Figure 1.
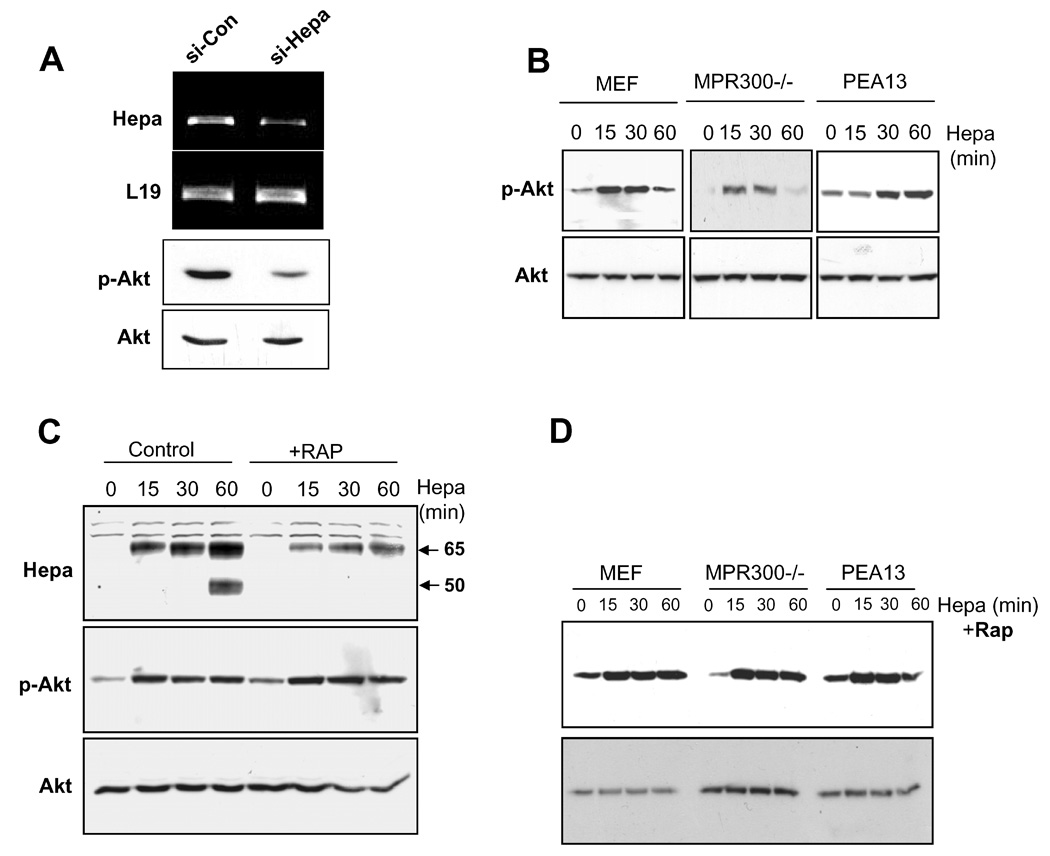
A. Enhanced Akt phosphorylation following heparanase addition is not mediated by MPR or LRP. A. Heparanase down regulation. B16/BL6 mouse melanoma cells were transfected with anti-heparanase (si-Hepa) or control (si-Con) si-RNA vectors, and heparanase expression was examined by RT-PCR analysis (upper panel). The expression of L19 (second panel) was used as an internal control. Corresponding cell lysates were subjected to immunoblotting applying anti-phospho-Akt (p-Akt, third panel) and total Akt (lower panel) antibodies. B. Control mouse embryonic fibroblasts (MEF), and fibroblasts deficient for MPR (MPR300−/−) or LRP (PEA13) were left untreated (0) or incubated (37°C) with heparanase (1 µg/ml) for the time indicated (min). Cell lysates were then prepared and subjected to immunoblotting with anti phospho-Akt (p-Akt, upper panels) and anti-Akt (Akt, lower panels) antibodies. C. RAP treatment. MEF or were left untreated (Control) or incubated with RAP (10 µg/ml, +RAP) for 15 min. Heparanase (1 µg/ml) was then added for the time indicated and cell lysates were subjected to immunoblotting with anti-heparanase (Hepa, upper panel), anti-phospho Akt (middle panel) and total Akt (lower panel) antibodies. D. MEF, MPR300−/−, and PEA13 cells were treated with RAP as above and heparanase (1 µg/ml) was then added for the time indicated (min). Total cell lysates were subjected to immunoblotting with anti-phospho Akt (upper panel) and anti-Akt (lower panel) antibodies.
