Figure 4.
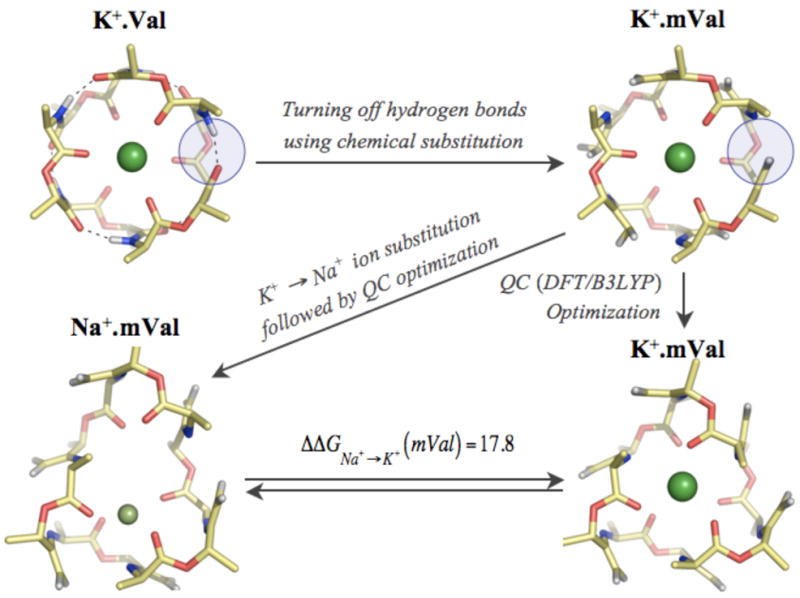
Structural and energetic effects of turning off the six intra-molecular hydrogen bonds in valinomycin. The hydrogen bonds, represented as dashed lines, were turned off using chemical substitutions involving replacement of proton acceptor atoms =O with =CH2 groups. A representative substitution is highlighted inside blue circles. QC optimization following these substitutions resulted in a K+ complexed structure K+.mVal with a backbone atom RMSD of 0.65 Å, and a K+-O distance of 2.88 Å. In the case of Na+ complex, Na+.mVal, these substitutions resulted in 4 of the 6 carbonyl oxygens to coordinate Na+ ions at smaller distances of 2.33 Å, as compared to 2.64 Å in the native Na+.Val structure. The remaining two carbonyl oxygens are at a distance of 5.12 Å from the Na+ ion. The absence of hydrogen bonds also resulted in increasing the free energy difference between the Na+ and K+ complexes from 12.3 to 17.8 kcal/mol.
