Figure 3. The NotI metal binding domain.
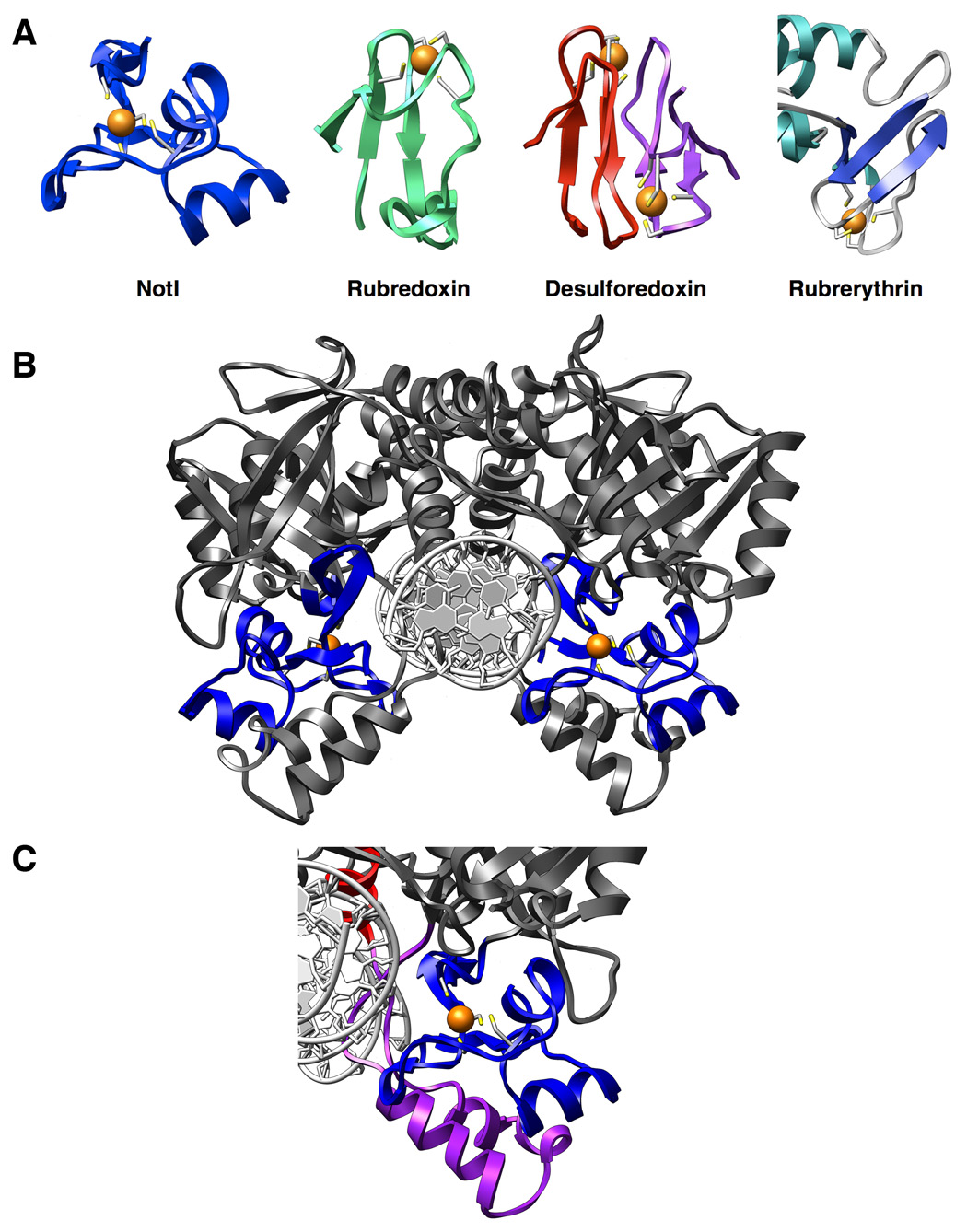
Panel A: FeCys4 Proteins - Rubredoxin (6rxn), desulforedoxin (1dxg), and rubrerythrin (1ryt) are three redox enzymes which contain the Fe-Cys4 iron binding motif, often referred to as the “rubredoxin-type” fold. Panel B: Homodimeric view of NotI with metal-binding domains highlighted in blue. Bound irons are shown as orange spheres and coordinating cysteine residues are colored by element and represented as sticks. The metal-binding domain is sandwiched between the nuclease core of the protein and the clamp domain (see panel c). Panel C: Iron-binding domain (blue) flanked by the clamp domain (purple). The iron-binding domain appears to serve an essential structural role in positioning of the clamp domain for contact with the DNA. The base of the DNA recognition helix making additional contacts to the DNA is also shown (red).
