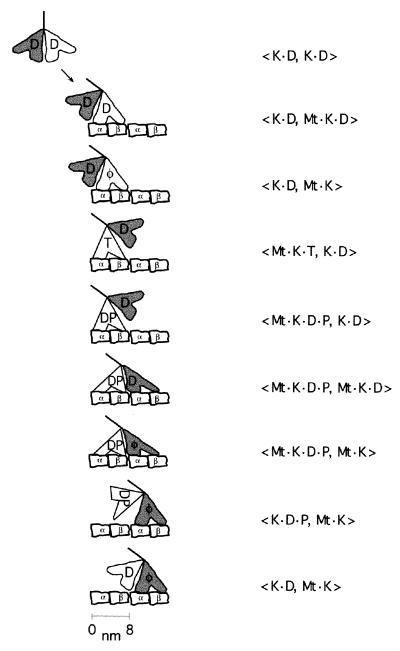
Figure 4.
Proposed two-headed hydrolysis cycle for kinesin. K, kinesin; Mt, microtubule. α and β are tubulin subunits; nucleotide abbreviations are same as in Fig. 3. The order of terms in the brackets denotes the position of the two heads along the microtubule from the minus- to the plus-end. See text for a detailed justification for this sequence of steps. Structural correlates for various states are as follows. (i) The 〈K⋅D, K⋅D〉 state corresponds to the crystal structure of homodimeric kinesin in the absence of microtubules (33). (ii) The 〈K⋅D, Mt⋅K⋅D〉 and the 〈K⋅D, Mt⋅K〉 states correspond to cryoEM reconstructions of microtubules decorated with kinesin in saturating ADP (47) and no nucleotide (47, 48), respectively, both of which show the detached head trailing the attached head in a poor orientation for microtubule binding. (iii) The 〈Mt⋅K⋅T, K⋅D〉 and 〈Mt⋅K⋅D⋅P, K⋅D〉 states correspond to the cryo-electron microscopic structures in AMP-PNP (48, 49) and in 1 mM ADP and 10 mM phosphate buffer (48, 50), respectively, in which the free head is situated closer to the next tubulin binding site.