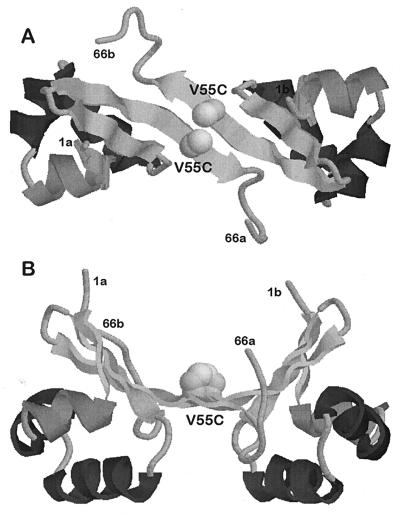
Figure 1.
Structure of the λCro repressor V55C variant (Val in position 55 replaced by Cys), based on the structure of the wild-type dimer. B shows a 90° rotation of A. The sites of amino acid substitution are indicated by spheres. The orientation of the two cysteine residues in Cro V55C allows the formation of a disulfide bridge. Coordinates for the wild-type protein were taken from Matsuo et al. (5).