Fig. 3.
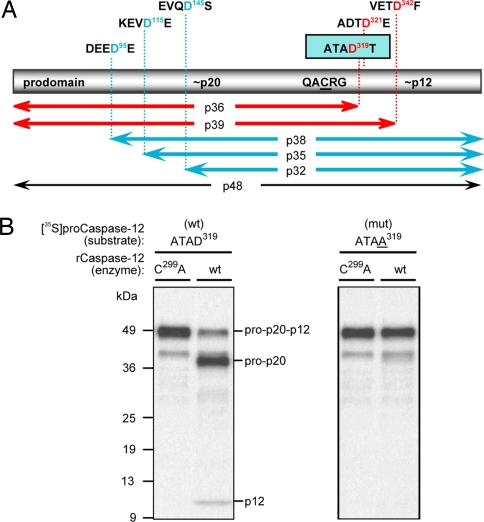
Identification of procaspase-12 autoprocessing site. (A) The rat caspase-12 proenzyme has a predicted molecular mass of 48 kDa and is represented by the bar with its tripartite constituents: the N-terminal prodomain, the 20-kDa large subunit that harbors the catalytic Cys residue within the QACRG pentapeptide motif, and the 12-kDa small subunit. Potential caspase cleavage motifs are identified above the bar, with the corresponding fragment sizes indicated below the bar. (B) [35S]procaspase-12 mutants were used as substrates for in vitro cleavage assays with bacterial lysates containing either the inactive form of caspase-12 (C299A; left lanes) or the wild-type form (right lanes). Only the conversion of the ATAD319 (Left) to ATAA319 (Right) resulted in the loss of autoprocessing activity.