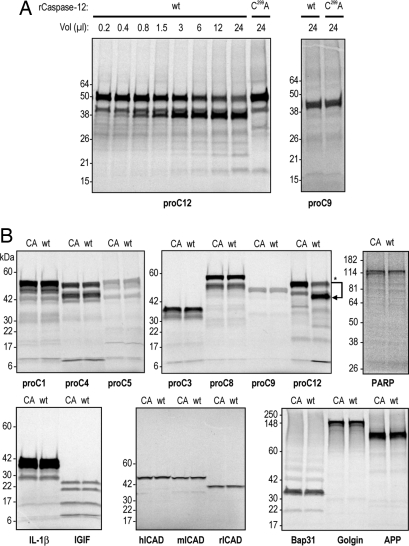
Fig. 4.
Macromolecular cleavage by caspase-12 does not extend to other known caspase substrates. Bacterial lysates containing either the inactive form of caspase-12 (C299A or CA) or the autoprocessed wild-type form of caspase-12 (wt) were used as enzyme for in vitro processing of the indicated [35S]precursor proteins. (A) Recombinant caspase-12 (rCaspase-12) titration shows concentration responsiveness in the reaction mixtures (Left), and the maximum concentration was used to test for rCaspase-12 processing of procaspase-9 (proC9) (Right). (B) Multiple caspase substrates were tested by the same procedure as described for A, with incubation times of 90 min. Only procaspase-12, used as a positive control, was cleaved.