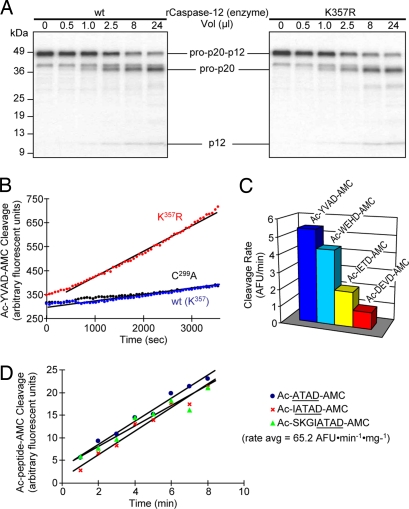
Fig. 5.
Activity of caspase-12 and K357R-modified caspase-12 on fluorogenic synthetic substrates. (A) Recombinant caspase-12 was generated by expression in bacteria and tested for cleavage of [35S]procaspase-12 (C299A) in vitro by using the indicated volumes of either wild-type caspase-12 (Left) or the K357R-modified caspase-12 (Right). No difference in cleavage was observed. (B) Various forms of recombinant caspase-12 were tested for activity against the canonical caspase-1 substrate, Ac-YVAD-AMC. No activity was detected with wild-type or catalytically incapacitated C299A caspase-12, whereas the K357R modification restored activity against this synthetic substrate. (C) The K357R-modified caspase-12 was tested for cleavage activity against a panel of fluorogenic substrates that are able to detect all known caspases. The preferred recognition pattern is consistent with other group-I caspases. (D) Indicated fluorogenic ligands corresponding to the autocleavage motif within procaspase-12 were prepared by solid-phase synthesis. Unmodified wild-type caspase-12 was expressed in bacteria and tested for fluorogenic activity against these and other fluorogenic ligands. Caspase-12 specific activity is 0.0652 AFU·min−1·mg−1.