Figure 7. ATP-dependent quenching of ACMA fluorescence by vacuolar membranes isolated from cells expressing wild type and fusion constructs.
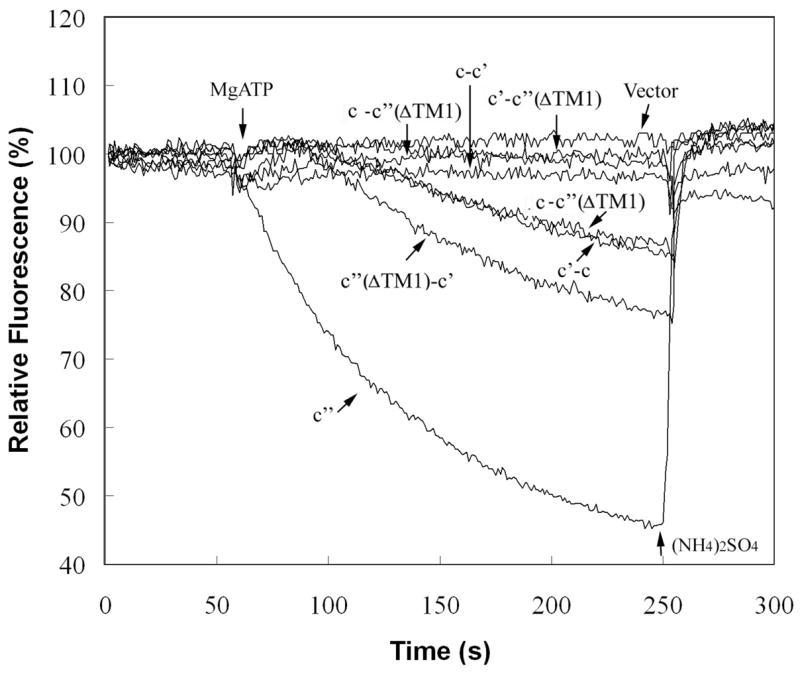
Vacuolar membranes (4 μg protein) isolated from cells expressing plasmid-borne proteolipids or proteolipid fusion constructs were assayed for ATP-dependent quenching of ACMA fluorescence as a measure of proton transport by addition of 1 mM MgATP and measurement of fluorescence intensity at 490 nm (excitation at 410 nm) as described under Experimental Procedures. The trace indicted by c″ corresponds to the activity observed for a vma16Δ strain expressing an HA-tagged form of subunit c″, whereas the remaining traces correspond to the activities measured for the fusion constructs expressed in the deletions strains indicated in Fig. 3. The activities were quantitated from the slopes of the traces and the values shown in Fig. 6 were corrected for any activity observed in the presence of 1 μM concanamycin, which was generally almost indistinguishable from the vector alone control. The slopes are proportional to the proton transport activity as indicated by the nearly linear relationship of the slope to the amount of vacuolar protein added (data not shown). At the indicated point, 5 mM (NH4)2SO4 was added to dissipate the pH gradient generated. It should be noted that the mutant strains give traces showing a significant lag before quenching following addition of MgATP, although the basis for this lag is not understood.
