Figure 3.
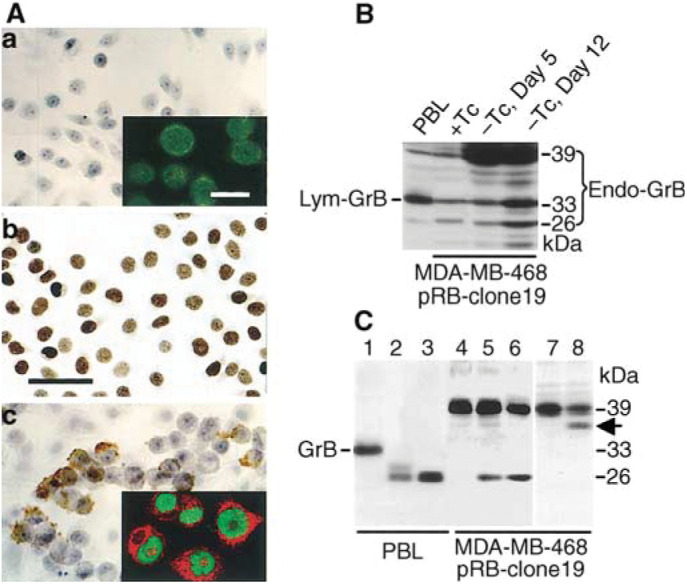
Characterization of endogenous GrB protein in RB-reconstituted MDA-MB-468 tumour cells. (A) Immunochemical staining of Endo-GrB (panels a and c) and pRB (panel b) of MDA-MB-468 pRB-clone 19-4. Endo-GrB was not detectable in tumour cells cultured in Tc-containing medium (Panel a), but was induced in Tc-free medium (Panel c). Tumour cells in Tc-free medium for 2 days exhibited uniformly pRB+ staining (Panel b). The CLSM images shown in the inserts of Panels a and c illustrate the double immunofluorescence staining of pRB (FITC, green) and Endo-GrB (Texas Red). Scale bars, 25 μm (12.5 μm in insets). (B) Western blotting. Endo-GrB protein triplets with molecular weights of 26, 33, 39 kDa were accumulated in RB-reconstituted cells grown in Tc-free medium. (C) The deglycosylated Endo-GrB and Lym-GrB proteins are identical in apparent molecular masses. Cell lysates were prepared from IL-2-activated PBL or MDA-MB-468 pRB-clone 19 cells (in Tc-free medium, Day 5). Each lane contains 5 μg of total cellular proteins treated with (lanes 1 and 4) reaction buffer only, and (lanes 2, 3, 5 and 6) with Endo H. Cell extracts in lanes 3 and 6 were predenatured. Following deglycosylation, both the 33-kDa mature Lym-GrB protein (lane 1) and the 39-kDa Endo-GrB protein (lanes 4) migrated to the identical position with an apparent Mr of 26 kDa (lanes 2, 3, 5 and 6). Also note that when small amounts of total cellular proteins (5 μg) were loaded in each lane, only the major species, that is, the 33-kDa glycosylated Lym-GrB in lane 1 and the 39-kDa glycosylated Endo-GrB in lane 4 were visible prior to Endo H treatment. (Lanes 7 and 8) The RB-reconstituted MDA-MB-468 cells were cultured in the absence (lane 7) or presence (lane 8) of tunicamycin. Arrow indicates a partially deglycosylated Endo-GrB of ∼36 kDa.
