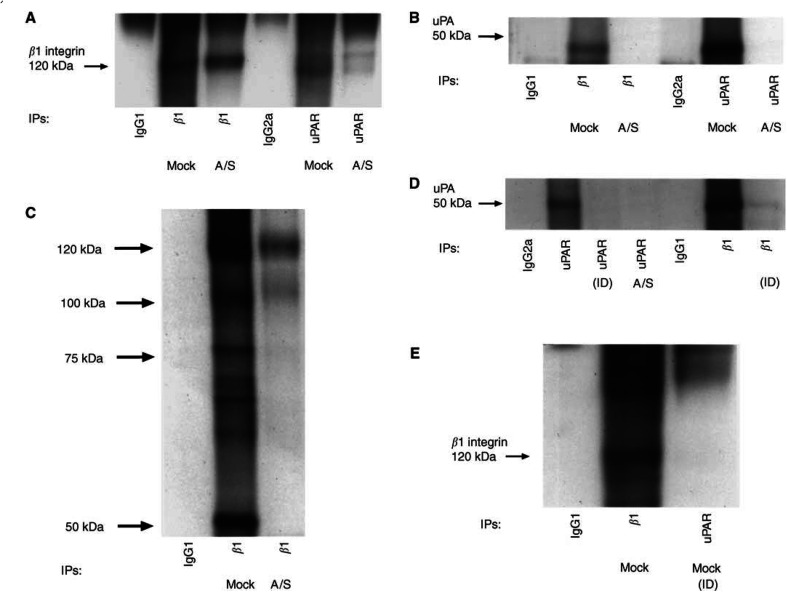
Figure 5.
Coimmunoprecipitation of β1 integrin and uPAR. Mock- and A/S-transfected HCT116 cells were lysed in Triton X-100 buffer (see Materials and Methods). Cell protein (500 μg) was mixed with anti-β1 antibody (PD52) or anti-uPAR antibody (3936) or isotype-matched mouse IgG and the resulting immunoprecipitates were analysed by Western blotting with (A) anti-β1-integrin antibody (PD52) or (B) anti-uPAR antibody (3936). (C) Cells were surface biotinylated and cell extracts were subjected to immunoprecipitation with anti-β1 antibody or with isotype-matched IgG antibodies and analysed by streptavidin–HRP binding to biotinylated proteins after SDS–PAGE and transfer to nitrocellulose membranes. (D and E) Mock-transfected cell lysates were immunodepleted (ID)of β1 after five rounds of sequential β1 and uPAR immunoprecipitation. Cell lysates were resolved by 10% SDS–PAGE followed by blotting with (D) anti-uPAR and (E) anti-β1 integrin antibody. The experiments were repeated at least three times.