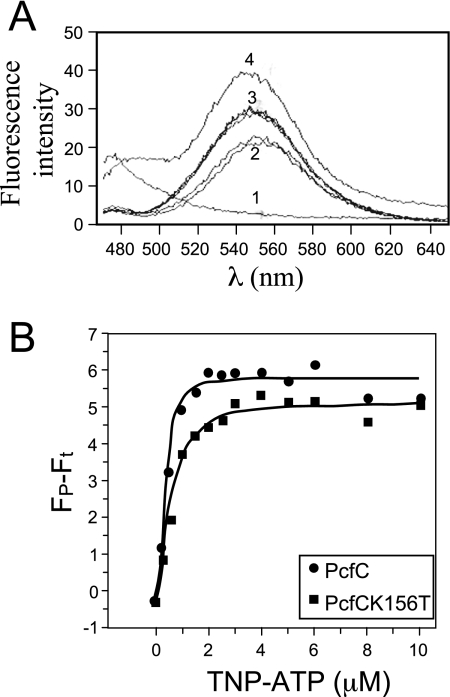
FIG. 6.
His6-PcfCΔN103 binds the fluorescent ATP analogue TNP-ATP. (A) Fluorescence was measured with an excitation wavelength of 410 nM and scanning wavelengths from 470 to 650 nM. Spectra 1 to 4 were taken from the following samples: 1, Purified His-PcfCΔN103 (2 μM) plus buffer; 2, TNP-ATP (30 μM) plus buffer (duplicate results are shown); 3, His-PcfCΔN103 (2 μM) plus TNP-ATP (30 μM) plus ATP (10 mM) (duplicate results are shown); 4, His-PcfCΔN103 (2 μM) plus TNP-ATP (30 μM). The spectrum obtained for His-PcfCΔN103K156T (2 μM) plus TNP-ATP (30 μM) closely matched that for His-PcfCΔN103 (data not shown). (B) Binding of TNP-ATP to WT and mutant PcfCΔN103. Concentration-dependent increases in TNP-ATP fluorescence in the presence of PcfCΔN103 (λ) and mutant K156T (ν) (1.5 mM each) indicate binding of this ATP analog to PcfC. The apparent fluorescence (Fp − Ft) is represented in arbitrary units after subtraction of intrinsic TNP-ATP fluorescence in buffer alone. The solid lines show the best fit of the Hill model (Origin program), giving Kds of 0.41 mM and 0.57 mM for WT PcfCΔN103 and mutant K151T, respectively.