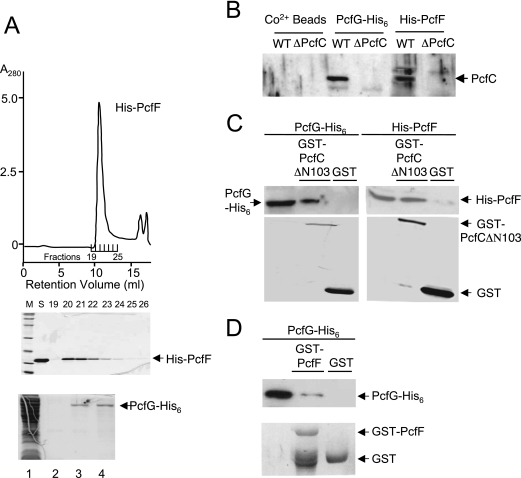
FIG. 7.
His6-PcfF purification, PcfG-His6 enrichment, and in vitro protein-protein interactions. (A) Recombinant His6-PcfF was purified by sequential affinity and gel filtration chromatographies (see text). Upper panel, gel filtration elution profile showing protein in peak fractions corresponding to a molecular size of ∼60 kDa. Middle panel, Coomassie blue-stained gel showing relative amounts of His6-PcfF protein in the fractions listed. Bottom panel, PcfG-His6 enrichment by affinity chromatography. Lanes: 1, total extract from PcfG-His6-producing E. coli; 2, final wash fraction; 3 and 4, first two fractions upon elution with 150 mM imidazole. (B) Binding of native PcfC to His-PcfF and PcfG-His6-CO2+ bead complexes. His-tagged proteins prebound to the CO2+ beads were mixed with E. faecalis cell extracts, and PcfC bound to the Pcf protein-bead complexes was identified as described in the text. (C) Pull-down assays using purified His-tagged PcfF and PcfG and GST-tagged PcfCΔN103. (D) Pull-down assays using purified PcfG-His6 and GST-PcfF.