Abstract
Streptococcus pneumoniae was shown to possess lactate oxidase in addition to well-documented pyruvate oxidase. The activities of both H2O2-forming oxidases in wild-type cultures were detectable even in the early exponential phase of growth and attained the highest levels in the early stationary phase. For each of these oxidases, a defective mutant was constructed and compared to the parent regarding the dynamics of pyruvate and lactate in aerobic cultures. The results obtained indicated that the energy-yielding metabolism in the wild type could be best described by the following scheme. (i) As long as glucose is available, approximately one-fourth of the pyruvate formed is converted to acetate by the sequential action of pyruvate oxidase and acetate kinase with acquisition of additional ATP; (ii) the rest of the pyruvate is reduced by lactate dehydrogenase to form lactate, with partial achievement of redox balance; (iii) the lactate is oxidized by lactate oxidase back to pyruvate, which is converted to acetate as described above; and (iv) the sequential reactions mentioned above continue to occur as long as lactate is present. As predicted by this model, exogenously added lactate was shown to increase the final growth yield in the presence of both oxidases.
The gram-positive bacterium Streptococcus pneumoniae, also known as pneumococcus, is often a commensal resident of the human upper respiratory tract but also represents an important human pathogen, causing invasive diseases such as pneumonia, otitis media, and meningitis (18, 20, 22). Recently, a sharp rise in the incidence of drug resistance among clinical isolates of S. pneumoniae has been posing serious problems (13).
A member of lactic acid bacteria, S. pneumoniae is aerotolerant but lacks the cytochromes necessary for aerobic respiration. Under anaerobic conditions, it is believed to be totally dependent on homolactic fermentation for the acquisition of energy required for growth, in which glucose is metabolized to pyruvate and then to the final product lactate. Under aerobiosis, however, pyruvate is also known to be converted to acetate, with acetyl phosphate being the intermediate capable of phosphorylating ADP to yield ATP by the action of acetate kinase (30). The H2O2-forming flavoprotein pyruvate oxidase (EC 1.2.3.3, the product of the spxB gene), which catalyzes the formation of acetyl phosphate, CO2 and H2O2 from pyruvate, orthophosphate, and O2, has been shown to be involved in this pathway and also to account for most of the H2O2 produced by aerobically growing S. pneumoniae cells (23, 30). Massive production of H2O2 has been a well-known hallmark of this bacterium since the time of Oswald Avery (2, 19), usually necessitating the addition of catalase to the culture medium to obtain full growth under aerobic conditions.
S. pneumoniae possesses another H2O2-forming flavoprotein, l-lactate oxidase (the product of the lox gene; formerly EC 1.1.3.2 but now sharing the EC number 1.13.12.4 with lactate monooxigenase) catalyzing the formation of pyruvate and H2O2 from l-lactate and O2. This enzyme was first described in this organism in 1959 (34), and its existence has recently been confirmed by the genomic information of this organism (33). Similar enzymes are now known in several other species of lactic acid bacteria, including S. pyogenes (29) and Lactobacillus plantarum (21, 26), and their likely involvement in aerobic lactate utilization has been documented.
In the present study, we present data indicating that, in S. pneumoniae, lactate oxidase converts lactate, usually regarded as a dead-end product of glucose metabolism in this organism, back to pyruvate, which is then subject to oxidation by pyruvate oxidase to form acetyl phosphate. Based on this finding, we propose that the two H2O2-producing oxidases act in a concerted manner in the presence of oxygen to obtain a greater amount of energy from glucose than under anaerobiosis, with acetate rather than lactate being the final product of the system.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in the present study are listed in Table 1. In most experiments with S. pneumoniae, cultures were grown at 37°C with gentle shaking in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) containing 0.2% glucose as an ingredient of product according to the manufacturer's information. For overnight cultures, the medium surface was covered with liquid paraffin to avoid H2O2 production. For experiments, the cells from such cultures were harvested by centrifugation at 4,000 × g for 5 min, washed twice with phosphate-buffered saline (PBS; pH 7.0), and suspended in an appropriate volume of fresh BHI broth to give a turbidity of 0.01 to 0.1 at 600 nm. When necessary, a filter-sterilized solution of bovine liver catalase (Sigma, St. Louis, MO) in PBS was added to the culture medium at a final concentration of 200 U/ml. When the effect of exogenous lactate on bacterial growth was studied, TYG medium (pH 7.4) containing 1% tryptose (Difco), 0.2% yeast extract (Difco), 0.5% NaCl, and 0.1% glucose was used as basal medium. Other culture media used in special circumstances are described in relevant sections below.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype and/or relevant informationa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | supE44 lacU169 (80lacZM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 8 |
| S. pneumoniae | ||
| GTC13809 | Wild-type encapsulated (serotype 19) | Gifu University |
| HT1 | spxB::Kanr derivative of GTC13809 | This study |
| HT2 | lox::Spcr derivative of GTC13809 | This study |
| Plasmids | ||
| pGEM-T Easy | Apr | Promega |
| pBRΩKm-2 | Kmr | 10 |
| pUC19 | Apr | Stratagene |
| pSET4s | lacZ′, Spr | 32 |
| pTN101 | pUC19 carrying lox fragment | This study |
| pTN102 | pGEM-T Easy carrying Spr cassette | This study |
| pTN103 | pTN101 carrying lox::Spr | This study |
| pTN201 | pGEM-T Easy carrying spxB fragment | This study |
| pTN202 | pTN201 carrying spxB::Kmr | This study |
Apr, ampicillin resistance; Spcr and Spr, spectinomycin resistance; Kanr and Kmr, kanamycin resistance.
Construction of mutants.
The nucleotide sequences of primers for PCRs are given in Table 2. The PCR primers used to amplify the spxB and lox genes were designed on the basis of the genomic sequence of strain TIGR4 (NC_003028). The primers for spxB were Spx1 and Spx2, but the EcoRI and BamHI adapters attached to their 5′ ends were not utilized eventually because the PCR product was inserted into pGEM-T Easy by the TA cloning technique to generate pTN201. The kanamycin resistance cassette (Kmr) was excised from pBRΩKm-2 with SmaI digestion. The primers for lox were Lox1 and Lox2, and the PCR product was inserted into the cloning site of pUC19 to generate pTN101. The spectinomycin resistance cassette (Spr) was amplified from pSET4s with primers Sp1 and Sp2, and the product was ligated to the SphI site of pGEM-T Easy to give pTN102. The cassette was then excised from pTN102. The enzymes used were purchased as follows: Ex Taq DNA polymerase and T4 DNA ligase were from Takara Bio (Shiga, Japan), and EcoRI, HindIII, HpaI, ScaI, SphI, and SmaI were from Toyobo (Osaka, Japan). All enzyme reactions were carried out as recommended by the suppliers. PCRs were performed with Ex Taq polymerase in the T1 thermocycler (Biometra, Goettingen, Germany), and purification of PCR products was carried out by DNA and gel band purification kit (GE Healthcare, Buckinghamshire, United Kingdom). Extraction and purification of plasmids were carried out by using a Wizard Plus kit (Promega, Madison, WI). Competence of GTC13809 cells for transformation was induced by the method of Prudhomme et al. (25) using norfloxacin at a final concentration of 2.5 μg/ml, which corresponds to half the MIC. Transformation was performed with the ScaI-digested donor plasmids essentially as described previously (3). The resulting transformants were selected on 5% blood agar containing catalase (Sigma) at 200 U/ml and supplemented with 400 μg of kanamycin/ml or 100 μg of spectinomycin/ml for spxB or lox mutants, respectively. For selection and propagation of Escherichia coli, cells harboring plasmids were done with L broth or L agar containing 1% Polypepton (Daigo Eiyo, Osaka, Japan), 0.5% yeast extract (Difco), and 0.5% NaCl, which was supplemented with kanamycin (30 μg/ml), spectinomycin (50 μg/ml), or ampicillin (50 μg/ml) as needed. Southern blot hybridization to confirm the desired insertions was carried out by using conventional techniques: the genomic DNAs from the test strains were digested with ScaI, which cuts neither the genes in question nor the inserts, and the target fragments were detected with probes made by PCR amplification and labeled with digitonin by using a Dig High Prime kit (Roche Diagnostics GmbH, Mannheim, Germany.). The PCR primers used were Spx-pr1 and Spx-pr2 for spxB and Lox-pr1 and Lox-pr2 for lox.
TABLE 2.
PCR primers used
| Primer | Sequence (5′-3′)a | Descriptionb |
|---|---|---|
| Spx1 | GGAATTCCATAATCTTTTAGGAGTGGTTCG | Forward primer for spxB |
| Spx2 | CGGGATCCCGCAACAGCTCTTTGAGCTTCC | Reverse primer for spxB |
| Lox1 | CGGAATTCCGGCAGCATTTGGCTATATCGC | Forward primer for lox |
| Lox2 | CCCAAGCTTGGGATGGATTGTGACGGAGCTTG | Reverse primer for lox |
| Sp1 | ATGCATGCATGTTCGTGAATACATGTTATA | Forward primer for Spr cassette |
| Sp2 | ATGCATGCATGTTTTCTAAAATCTGAT | Reverse primer for Spr cassette |
| Spx-pr1 | GTTGTCGGAATTCCGATTGC | Forward primer for spxB probe |
| Spx-pr2 | CTTTGTCTTCAGCCAAAGCG | Reverse primer for spxB probe |
| Lox-pr1 | CGGAATTCCGGCAGCATTTGGCTATATCGC | Forward primer for lox probe |
| Lox-pr2 | GCGCAGTCGCCACTTCCCCC | Reverse primer for lox probe |
Underlined are restriction site adapters. Lox-pr1is identical to Lox1.
These primers were designed on the basis of the genome sequence of strain TIGR4 (accession no. NC_003028).
Spectrophotometric measurements.
A spectronic Genesys 5 spectrophotometer (Milton Roy, Rochester, NY) was used for all photometric measurements including turbidometry. The light path was 1 cm in length.
Assay of H2O2.
This was carried out by two different methods depending on the concentration of H2O2. The titanium sulfate assay, which is less sensitive but highly stable and specific, was performed by mixing a 60-μl aliquot of a sample with 0.6 ml of 5% titanium(IV) sulfate solution (Nacalai Tesque, Kyoto, Japan) in a plastic cuvette and reading the absorbance at 420 nm. A FOX I assay, which is suitable for determining μM levels of H2O2, was carried out as described previously (11).
Determination of glucose and its metabolic products in culture supernatants.
Culture samples, 1 ml each, were centrifuged at 16,000 × g for 5 min, and the supernatants were used for each assay. Glucose and acetate were determined by using a Wako Glu 2 kit (L-Type; Wako, Osaka, Japan) and an F-kit for acetic acid (Roche Diagnostics GmbH), respectively. Enzymatic determination of lactate was carried out with l-lactate dehydrogenase from rabbit muscle (Sigma) as described previously (9). Determination of H2O2 was done either by using the titanium sulfate assay or by using the FOX I assay.
Assay of pyruvate and lactate oxidase activities.
Cells were collected from appropriate volumes of cultures and washed twice with PBS by centrifugation at 16,000 × g for 5 min. The pelleted cells were resuspended in PBS to give a turbidity of 0.5 or 0.1 at 600 nm for the assay of pyruvate oxidase or lactate oxidase, respectively. The cells were then permeabilized by the method of Kornberg and Reeves (14) by mixing the suspensions with 0.01 volumes of toluene-ethanol (1:9 [vol/vol]) with a vortex mixer for 1 min. Pyruvate oxidase activity was assessed by assaying the acetyl phosphate produced essentially as described previously (7, 16). The reaction mixture consisted of 0.5 ml of the permeabilized cell suspension and 0.5 ml of a solution containing 50 mM potassium phosphate buffer (pH 6.0), 10 μM MgCl2, 0.2 μM thiamine pyrophosphate (Sigma), 50 mM potassium pyruvate, and 12 μM FAD (Sigma), and a reaction was initiated by the addition of enzyme. After being incubated at 37°C for 20 min with shaking, the reaction mixture received 1 ml each of 4 M hydroxylamine (pH 6.4) and 0.1 M acetate buffer (pH 5.4), and was kept standing for 10 min at room temperature. Then, 1 ml each of 36% HCl, 12% (wt/vol) trichloroacetic acid, and 5% (wt/vol) ferric chloride in 0.1 N HCl were added to the mixture, which was kept standing at room temperature for 20 min before being centrifuged at 16,000 × g for 5 min. The absorbance of the supernatant was measured at 540 nm. Lactate oxidase activity was assessed by assaying the H2O2 produced by a modification of the method previously described (29). The reaction mixture consisted of 1 ml of the permeabilized cell suspension and 3 ml of 0.1 M sodium phosphate buffer (pH 7.0) containing 2.2 mM sodium l-lactate (Sigma). The reaction was initiated by the addition of enzyme and incubated at 37°C with shaking. At 5-min intervals, 0.5-ml samples were withdrawn and immediately mixed with 60 μl of concentrated HCl in glass tubes chilled in an ice-water bath. The mixtures were centrifuged for 5 min at 16,000 × g, and a 50-μl portion of each supernatant was used for H2O2 determination by the FOX I method. The oxidase activity was calculated from the slope of the linear portion of each reaction.
RESULTS
H2O2 production by the wild-type strain.
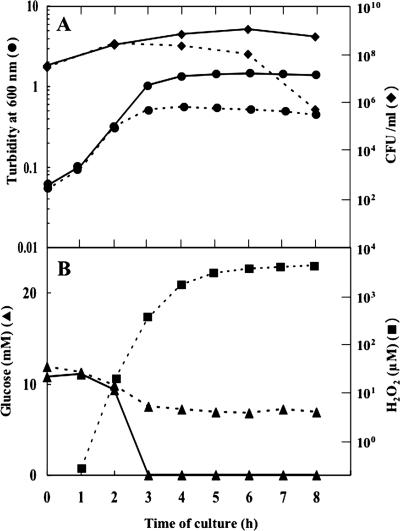
Under aerobic conditions, cultures of S. pneumoniae strains are known to accumulate unusually high concentrations of H2O2, which necessitates the addition of catalase to the medium for full growth (2, 19). We confirmed that S. pneumoniae strain GTC13809, a serotype 19 clinical isolate used throughout the present work, behaved as a typical wild type in this respect (Fig. 1). In BHI broth (glucose concentration, 11 mM) without added catalase, the concentration of H2O2 in the culture medium began to rise in the early exponential phase and attained a level of approximately 5 mM in the middle exponential phase. In the meantime, there occurred a premature cessation of cell growth and a large loss of viability (Fig. 1A). These cultural anomalies were prevented completely by the presence of catalase exogenously added in the medium.
FIG. 1.
Cultural characteristics of the wild-type GTC13809. Cultures were grown in BHI broth aerobically with (solid lines) or without (dotted lines) added catalase and examined with respect to various parameters. (A) Viability in CFU (⧫) and cell mass in turbidity (•); (B) levels of glucose (▴) and H2O2 (▪). Representative results of at least five independent experiments are shown.
The early onset of H2O2 production suggests that it is not under the control of a catabolite repression-like regulatory mechanism. Our results showed that this was actually the case; the production of H2O2 started when only part of the glucose in the medium had been consumed (Fig. 1B). Furthermore, the addition of a large excess of glucose (1%) to the culture medium did not alter the time course of H2O2 production (data not shown). This situation is different from that of S. pyogenes, in which H2O2 production is absent in glucose-supplemented medium (5) and starts only after the supply of glucose is exhausted (27, 29).
Activities of H2O2-forming oxidases.
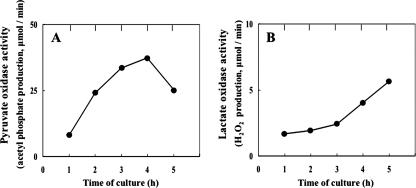
The massive production of H2O2 by S. pneumoniae has recently been ascribed to pyruvate oxidase, which catalyzes oxygen- and phosphate-dependent oxidation of pyruvate to form acetyl phosphate, CO2, and H2O2 (24, 30). Thus, these studies have clearly demonstrated that SpxB-deficient mutations drastically diminish H2O2 production by this bacterium. Using permeabilized cell preparations, we confirmed that our wild-type strain, GTC13809, manifested the activity of pyruvate oxidase, which was detectable even in the early exponential phase of growth and increased until the culture entered the late exponential phase (Fig. 2A). This profile of activity appears to be consistent with, and perhaps constitutes the basis of, the relationship between the growth phase and H2O2 production.
FIG. 2.
Growth-phase-dependent changes in the activity of H2O2-forming oxidases in strain GTC13809. (A) Pyruvate oxidase; (B) lactate oxidase. Cells were grown in BHI broth aerobically in the presence of exogenous catalase. The enzyme activity was determined with permeabilized cells as described in Materials and Methods. Representative results of at least five independent experiments are shown.
On the other hand, we became aware through a search against the genome database that S. pneumoniae possesses a gene whose putative product (NP_ 345216 for strain TIGR4 and NP_358221 for strain R6) is highly similar to S. pyogenes (accession number NP_268722 for strain M1GAS) and L. plantarum (NP_786785 [12]) in the amino acid sequence. Because the nomenclature has not yet been established for the lactate oxidase gene at large, we tentatively call the gene of S. pneumoniae lox for lactate oxidase.
To confirm the existence of this enzyme, we went on to examine cells of GTC13809 for its activity using permeabilized cells. The results obtained clearly showed such activity to exist, which was detectable even in cells from early-exponential-phase cultures and increased as growth progressed, as in the case of pyruvate oxidase (Fig. 2B).
Construction of oxidase-deficient mutants.
To define the physiological roles of the two oxidases more fully, we were in need of mutants deficient in each of them. Although an SpxB-defective mutant had previously been described (30), a Lox-defective mutant was yet to be isolated.
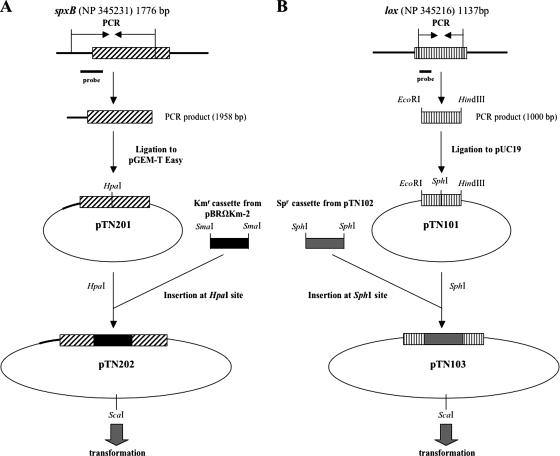
We constructed both mutants by introducing drug resistance cassettes into respective wild-type genes through genetic transformation (Fig. 3). For an spxB mutant, a 1,958-bp segment spanning from a site in the upstream region of the gene to an internal site near the 3′ end was amplified, and the product was introduced into pGEM-T Easy to make pTN201. The Kmr cassette from pBRΩKm-2 was inserted into the HpaI site of pTN201 to give pTN202, which was linearized by ScaI digestion and used for transformation. For a lox mutant, an internal 1,000-bp region of the gene was amplified with an EcoRI and a HindIII adapter attached to the 5′ and 3′ ends, respectively, and the amplified DNA was inserted into pUC19 to give pTN101. The Spr cassette from pSET4s was inserted into the SphI site of pTN101 to give pTN103, which was used in transformation following linearization with ScaI digestion.
FIG. 3.
Construction of oxidase-deficient mutants. The plasmids and primers used are listed in Tables 1 and 2, respectively.
Transformation was carried out with strain GTC13809 as the recipient, following competence induction with norfloxacin (25). One drug-resistant colony was chosen from each transformation as a candidate for the desired mutant and confirmed to carry the insertion within the spxB or lox gene by Southern hybridization (data not shown). The spxB and lox mutants thus obtained were named HT1 and HT2, respectively.
Cultural characteristics of the mutants.
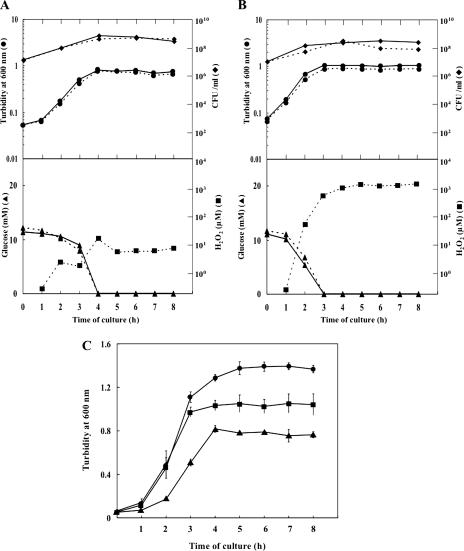
The mutants thus obtained were examined for their growth characteristics and H2O2 production (Fig. 4A and B). In the spxB mutant HT1, levels of H2O2 in the medium remained extremely low (≤10 μM) until the glucose was consumed completely, lending support to the notion that massive H2O2 production by wild-type cells can be accounted for by the activity of pyruvate oxidase (24, 30). As a result, the culture attained apparently full growth without added catalase. It must be mentioned, however, that the final cell mass (turbidity) attained by this mutant in the presence or absence of exogenous catalase was reproducibly lower by 0.5 to 0.6 turbidity unit than that reached by the parental strain in the presence of catalase (Fig. 4C).
FIG. 4.
Cultural characteristics of the oxidase-deficient mutants. Cultures were grown in BHI broth under aerobic conditions in the presence (solid lines) or absence (dotted lines) of exogenous catalase. (A) SpxB-defective mutant HT1; (B) Lox-defective mutant HT2. Upper panels show cell growth in terms of cell mass (•) and cell viability (⧫); lower panels show glucose consumption (▴) and H2O2 (▪). Representative results of at least five independent experiments are shown. (C) Comparison of the final levels of cell mass achieved by the wild type (•), the SpxB-defective mutant HT1 (▴), and the Lox-defective mutant HT2 (▪). Means ± the standard deviations for three independent experiments are shown.
In the lox mutant HT2, the highest concentration of H2O2 attained in the medium was found to be approximately 1 mM, about one-fifth the level for the parental strain. Although cell growth continued until glucose in the medium became undetectable even in the absence of added catalase, the maximum cell mass attained was considerably higher in the presence of catalase than in its absence, probably reflecting cell injury by the H2O2 formed. It was noted, as in the case of the spxB mutant, that the maximum cell mass achieved in the presence of added catalase was reproducibly lower by 0.2 to 0.3 turbidity unit than that of the parent (Fig. 4C).
Dynamics of metabolic products in culture supernatants.
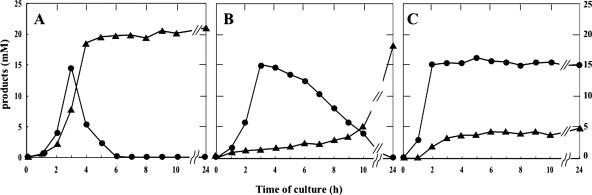
We have previously shown that lactate oxidase of S. pyogenes is responsible for the conversion of lactate to acetate via acetyl phosphate with concomitant generation of ATP (29). To investigate whether such utilization of lactate also takes place in S. pneumoniae, we studied the dynamics of lactate and acetate in cultures of the parental strain and the two oxidase-deficient mutants. The results may be summarized as follows.
In all strains examined, the periods during which lactate levels initially increased roughly coincided with those in which glucose in the medium was available (cf. Fig. 1 and 4). Thereafter, lactate levels decreased in the presence of lactate oxidase (Fig. 5A and B) or stayed constant in its absence, suggesting that lactate oxidase is involved in the reflux of lactate to pyruvate (Fig. 5C). Notably, the rate of the decrease in the lactate level was markedly slow in the absence of pyruvate oxidase (Fig. 5B). This may be a secondary effect of the pyruvate oxidase deficiency (see below). On the other hand, acetate levels increased with time in all test strains, but with different profiles. In the absence of lactate oxidase, the acetate level rose slowly and reached a plateau when glucose became unavailable (Fig. 5C). In this case, the final molar ratio of lactate to acetate was 3 to 1. We consider that this ratio could be regarded as reflecting the relative efficiencies of NADH-dependent lactate dehydrogenase and pyruvate oxidase with which to metabolize pyruvate. In the case of pyruvate oxidase deficiency (Fig. 5B), the acetate concentration rose very slowly and reached the final level of ∼20 mM after 24 h, which was comparable to the level attained in the wild type.
FIG. 5.
Dynamics of lactate and acetate concentrations. Cultures were grown in BHI broth under aerobic conditions with added catalase, and the levels of lactate (•) and acetate (▴) in the culture supernatant were monitored. (A) Wild type; (B) SpxB-defective mutant HT1; (C) Lox-defective mutant HT2.
The enzyme responsible for this extremely slow formation of acetate is currently unknown, but the pyruvate dehydrogenase complex is an obvious possibility. In the case of wild type, where both oxidases are at work (Fig. 5A), it is likely that a major pathway of pyruvate metabolism involves the sequential action of lactate dehydrogenase, lactate oxidase, pyruvate oxidase, and acetate kinase, while the direct oxidation of pyruvate to acetyl phosphate by pyruvate oxidase followed by acetate formation by acetate kinase constitutes a minor pathway (see above). From the results in Fig. 5C, the contribution of each pathways to ATP production would be estimated to be at a ratio of 3 to 1.
It would be pertinent to mention two different aspects of stoichiometry. One is that the sum of the final levels of lactate and acetate was about 20 mM in each of the three strains, approximately twice the initial concentration of glucose (∼11 mM). This indicates that the conversion of glucose to final products is practically quantitative. This is in accordance with the established fact that in nonrespiratory bacteria growing in rich medium, the fermentative substrate serves only as a source of energy and is not converted to cellular material (31). The other aspect is that there is no apparent stoichiometric relationship between the levels of the final products and that of H2O2. A typical example of such situations was seen in the spxB mutant: the level of H2O2 accumulated in the culture was stabilized at about 10 μm (Fig. 4A), but the decrease in the concentration of lactate amounted to almost 10 mM in the same period of time (Fig. 5B). A plausible explanation of such discrepancies would be that considerable portions of H2O2 formed by lactate oxidation must have been decomposed by the cellular scavenging system.
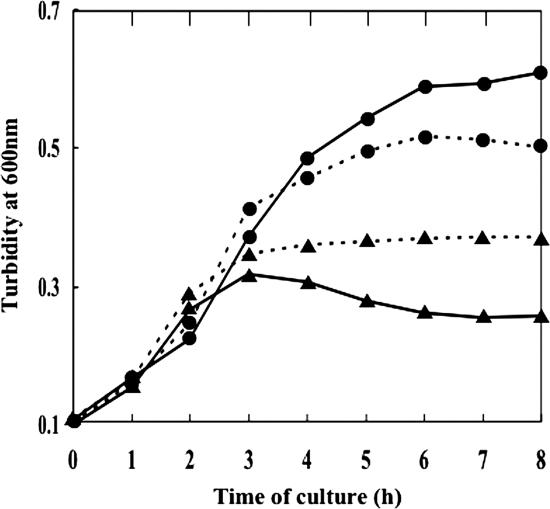
Effect of exogenous lactate on bacterial growth.
Since lactate oxidase is able to form pyruvate from lactate, cells of wild type, but not those of the lox mutant, might possibly utilize exogenously added lactate to acquire additional ATP, and hence extra cell mass, by metabolizing the pyruvate further to acetate via acetyl phosphate. To test this possibility, cultures were grown in TYG broth containing 0.1% glucose and exogenous catalase in the presence or absence of 0.5% sodium l-lactate (Fig. 6). In the case of GTC13809, the final cell mass in terms of turbidity of the culture was higher in the presence of sodium lactate than in its absence, thus substantiating the above possibility. In contrast, however, growth of the lox mutant was strongly repressed by lactate. Presumably, the protonated form of lactate, i.e., lactic acid, capable of permeating through the cell membrane easily, accumulated within the cell and exerted cytotoxicity in the absence of removal by the action of lactate oxidase.
FIG. 6.
Effect of exogenous lactate on bacterial growth. Cells of wild type (•) and Lox-defective mutant HT2 (▴) were cultured in TYG medium containing 0.1% glucose under aerobic conditions with (solid line) or without (dotted line) 0.5% sodium l-lactate added to the medium.
DISCUSSION
Of the two H2O2-producing oxidases found in the genomic sequence of S. pneumoniae, pyruvate oxidase or SpxB protein has been studied in some detail with respect to its roles in glucose metabolism, H2O2 resistance, and virulence (15, 24, 30). It is also known that the spxB gene is expressed constitutively, with little influence of growth phase (30). On the other hand, lactate oxidase or Lox protein, which was first documented in as early as 1959 in S. pneumoniae (34), has since been largely ignored. In the present work, we were able to show that not only the activity of pyruvate oxidase but also that of lactate oxidase was manifest even in the presence of excess glucose, indicating that the expression of the lox gene is not under the tight control of a catabolite repression-like regulatory mechanism either. These results are in sharp contrast to the situations of homologous proteins in other bacterial species: the activity of lactate oxidase in S. pyogenes (5, 29) and S. iniae (4) and that of pyruvate oxidase in L. plantarum (6, 17, 21, 26, 28) are all undetectable as long as glucose is available from the medium. Obviously, the practically constant expression of the spxB and lox genes represents a unique feature of S. pneumoniae.
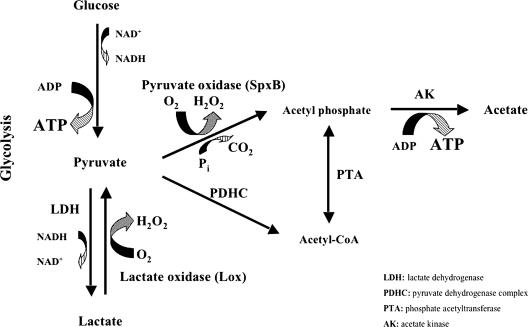
Characterization of the lox and spxB mutants isolated in the present study was helpful in clarifying the roles of these two oxidases in the aerobic metabolism of glucose. The time-dependent changes of the lactate and acetate levels observed in the three S. pneumoniae strains are considered to be best explained by the following scheme (Fig. 7). In wild-type cells aerobically growing on glucose, ca. 25% of the pyruvate in the pool is converted directly to acetyl phospate by pyruvate oxidase, while the rest makes a detour consisting of conversion to lactate by NADH-dependent lactate dehydrogenase and by lactate oxidase-mediated return to the pyruvate pool. In the end, the acetyl phosphate formed phosphorylates ADP to generate ATP and acetate by acetate kinase. Once glucose is exhausted, the remaining lactate becomes the sole energy source and is metabolized sequentially by lactate oxidase, pyruvate oxidase, and acetate kinase as mentioned above.
FIG. 7.
Proposed pathway of aerobic metabolism of glucose in S. pneumoniae.
One thing that needs further attention is the achievement of redox balance. In the scheme described above, the recycling of NADH is apparently incomplete because only part of the pyruvate derived from glucose is available for NADH oxidation by lactate dehydrogenase. We suggest that this difficulty can be overcome by assuming two possibilities. One is a cycling between pyruvate and lactate, in which a portion of the pyruvate pool accounted for by lactate oxidase-mediated reflux from lactate may be reduced back to lactate again by lactate dehydrogenase with concomitant oxidation of NADH. The other possibility is the involvement of NADH oxidase, the presence of which has been documented (1).
It would be pertinent to emphasize that the system of S. pneumoniae described above is featured by the independence from the growth phase or the presence of glucose. In all other lactic acid bacteria thus far examined, the manifestation of lactate or pyruvate oxidase activity is absent as far as glucose is available from the medium (4, 6, 17, 21, 26, 28, 29). Another feature is that S. pneumoniae has pyruvate oxidase, as well as lactate oxidase, which distinguishes it from S. pyogenes possessing only lactate oxidase. In this sense, S. pyogenes resembles the spxB mutant of S. pneumoniae.
Finally, the merit of this system for the invasive pathogen may not be limited to the high efficiency of energy acquisition, since acetyl phosphate and H2O2 have been directly implicated in the virulence of this organism (30). A prerequisite for this argument would be that cells of S. pneumoniae can tolerate H2O2 massively produced by themselves at the site of infection. We surmise that diffusion and/or decomposition of H2O2 are quick enough to fill this requirement.
In conclusion, aerobiosis makes the concerted action of lactate oxidase and pyruvate oxidase possible, enabling cells of S. pneumoniae to gain more ATP from glucose than under anaerobiosis.
Acknowledgments
We are grateful to Yoshiaki Kawamura (Aichi Gakuin University) for providing S. pneumoniae strains. We thank Hideko Kajiwara, for technical assistance, as well as Takashi Soejima (Morinaga Milk Industry) and Eiji Harada for useful suggestions. H.T. is particularly indebted to Jun Hayashi for permission to join the present work. We thank L. Saza for her helpful comments on the English of an earlier version of the manuscript.
Footnotes
Published ahead of print on 14 March 2008.
REFERENCES
- 1.Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 341018-1028. [DOI] [PubMed] [Google Scholar]
- 2.Avery, O. T., and H. J. Morgan. 1924. The occurrence of peroxide in cultures of pneumococcus. J. Exp. Med. 39275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bricker, A. L., and A. Camilli. 1999. Transformation of a type 4 encapsulated strain of Streptococcus pneumoniae. FEMS Microbiol. Lett. 172131-135. [DOI] [PubMed] [Google Scholar]
- 4.Gibello, A., M. D. Collins, L. Dominguez, J. F. Fernandez-Garayzabel, and P. T. Richardson. 1999. Cloning and analysis of the l-lactate utilization genes from Streptococcus iniae. Appl. Environ. Microbiol. 654346-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson, C. M., T. C. Mallett, A. Claiborne, and M. G. Caparon. 2000. Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goffin, P., L. Muscariello, F. Lorquet, A. Stukkens, D. Prozzi, M. Sacco, M. Kleerebezem, and P. Hols. 2006. Involvement of pyruvate oxidase activity and acetate production in the survival of Lactobacillus plantarum during the stationary phase of aerobic growth. Appl. Environ. Microbiol. 727933-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hager, L. P., D. M. Geller, and F. Lipmann. 1954. Flavoprotein-catalyzed pyruvate oxidation in Lactobacillus delbrueckii. Fed. Proc. 13734-738. [PubMed] [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 9.Hohorst, H. J. 1963. Determination with lactic dehydrogenase and DPN, p. 266-270. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis. Academic Press, Inc., New York, NY.
- 10.Jadoun, J., O. Eyal, and S. Sela. 2002. Role of CsrR, hyaluronic acid, and SpeB in the internalization of Streptococcus pyogenes M type 3 strain by epithelial cells. Infect. Immun. 70462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, Z. Y., A. C. Woollard, and S. P. Wolff. 1991. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange: comparison with the TBA assay and an iodometric method. Lipids 26853-856. [DOI] [PubMed] [Google Scholar]
- 12.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 1001990-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klugman, K. P. 2007. Clinical impact of antibiotic resistance in respiratory tract infections. Int. J. Antimicrob. Agents 29(Suppl. 1)S6-S10. [DOI] [PubMed] [Google Scholar]
- 14.Kornberg, H. L., and R. E. Reeves. 1972. Correlation between hexose transport and phosphotransferase activity in Escherichia coli. Biochem. J. 1261241-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeMessurier, K. S., A. D. Ogunniyi, and J. C. Paton. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology 152305-311. [DOI] [PubMed] [Google Scholar]
- 16.Lipmann, F., and L. C. Tuttle. 1945. A specific micromethod for the determination of acyl phosphates. J. Biol. Chem. 15921-28. [Google Scholar]
- 17.Lorquet, F., P. Goffin, L. Muscariello, J. B. Baudry, V. Ladero, M. Sacco, M. Kleerebezem, and P. Hols. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J. Bacteriol. 1863749-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason, E. O., Jr., E. R. Wald, T. Q. Tan, G. E. Schutze, J. S. Bradley, W. J. Barson, L. B. Givner, J. Hoffman, and S. L. Kaplan. 2007. Recurrent systemic pneumococcal disease in children. Pediatr. Infect. Dis. J. 26480-484. [DOI] [PubMed] [Google Scholar]
- 19.McLeod, J. W., and J. Gordon. 1922. Production of hydrogen peroxide by bacteria. Biochem. J. 16499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mufson, M. A. 1999. Pneumococcal pneumonia. Curr. Infect. Dis. Rep. 157-64. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, M. G., L. O'Connor, D. Walsh, and S. Condon. 1985. Oxygen-dependent lactate utilization by Lactobacillus plantarum. Arch. Microbiol. 14175-79. [DOI] [PubMed] [Google Scholar]
- 22.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14801-807. [DOI] [PubMed] [Google Scholar]
- 23.Pericone, C. D., K. Overweg, P. W. Hermans, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 683990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 1856815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prudhomme, M., L. Attaiech, G. Sanchez, B. Martin, and J. P. Claverys. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 31389-92. [DOI] [PubMed] [Google Scholar]
- 26.Quatravaux, S., F. Remize, E. Bryckaert, D. Colavizza, and J. Guzzo. 2006. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 101903-912. [DOI] [PubMed] [Google Scholar]
- 27.Saito, M., S. Ohga, M. Endoh, H. Nakayama, Y. Mizunoe, T. Hara, and S. Yoshida. 2001. H2O2-nonproducing Streptococcus pyogenes strains: survival in stationary phase and virulence in chronic granulomatous disease. Microbiology 1472469-2477. [DOI] [PubMed] [Google Scholar]
- 28.Sedewitz, B., K. H. Schleifer, and F. Götz. 1984. Physiological role of pyruvate oxidase in the aerobic metabolism of Lactobacillus plantarum. J. Bacteriol. 160462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seki, M., K. Iida, M. Saito, H. Nakayama, and S. Yoshida. 2004. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate. J. Bacteriol. 1862046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19803-813. [DOI] [PubMed] [Google Scholar]
- 31.Stanier, R. Y., E. A. Adelberg, and J. Ingraham. 1976. The microbial world. 4th ed, p.284. Prentice-Hall, Englewood Cliffs, NJ.
- 32.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46140-148. [DOI] [PubMed] [Google Scholar]
- 33.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293498-506. [DOI] [PubMed] [Google Scholar]
- 34.Udaka, S., J. Koukol, and B. Vennesland. 1959. Lactic oxidase of pneumococcus. J. Bacteriol. 78714-725. [DOI] [PMC free article] [PubMed] [Google Scholar]