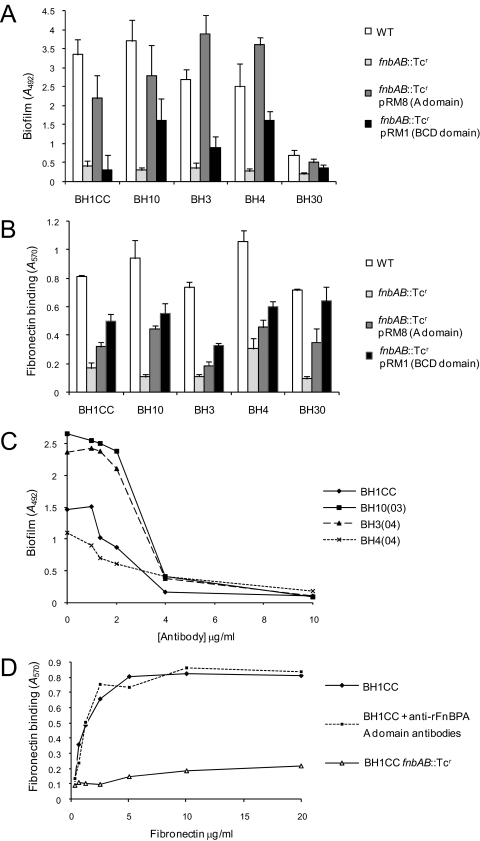
FIG. 8.
Role of FnBPA structural domains in MRSA biofilm development and binding to immobilized human fibronectin. (A) Complementation of biofilm development in MRSA fnbAB::Tcr mutants by the A (pRM8) or BCD (pRM1) domains of the FnBPA protein. MRSA isolates BH1CC, BH10, BH3, BH4, and BH30 were grown in BHI medium supplemented with 1% glucose at 37°C for 24 h. (B) Complementation of fibronectin binding activity in MRSA fnbAB::Tcr mutants by the A (pRM8) or BCD (pRM1) domains of the FnBPA protein. Cells of BH1CC, BH10, BH3, BH4, and BH30 were tested for their ability to bind to immobilized human fibronectin (5 μg/ml). (C) Inhibition of MRSA biofilm development by anti-FnBPA37-544 A-domain antibodies. MRSA isolates BH1CC, BH10, BH4, and BH3 were grown in BHI medium supplemented with 1% glucose and various concentrations of polyclonal anti-FnBPA A-domain antibodies at 37°C for 24 h. Values represent the mean of triplicate wells. (D) Binding of the MRSA isolate BH1CC to immobilized human fibronectin in the presence and absence of polyclonal anti-FnBPA A-domain antibodies. BH1CC fnbAB::Tcr was used as a negative control. Values represent the mean of triplicate wells.