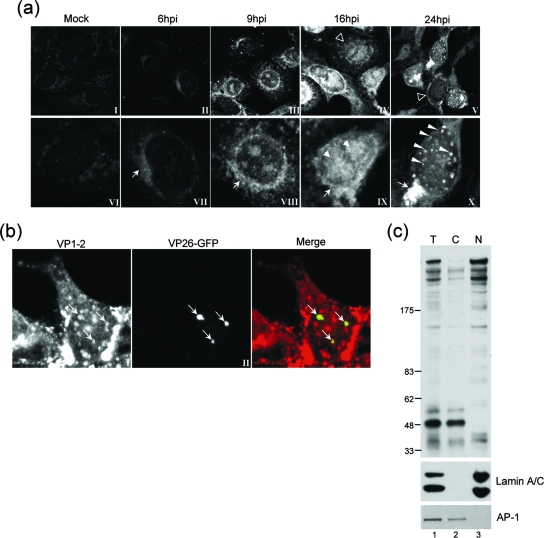
FIG. 2.
Subcellular localization and fractionation of VP1-2 during virus infection. (a) Monolayers of Vero cells grown on coverslips were mock infected (panels I and VI) or infected with the HSV-1 17 strain (panels II to V and VII to X). At different times postinfection, coverslips were washed, fixed, and probed for VP1-2 using αVP1-2NT1r. Lower panels show higher-magnification images of the top row. Features of VP1-2 localization as discussed in the text are illustrated with different arrows, as follows: cytoplasmic perinuclear signal, arrow, e.g., panels VII and VIII; diffuse intranuclear localization, filled arrowhead, panel IX; nuclear punctate, long arrowheads. Cells exhibiting a diffuse localization are indicated by open arrowheads in the lower-magnification images in panels IV and V. (b) Monolayers of Vero cells were infected with a recombinant virus HSV-1 strain 17 expressing VP26-GFP and cells examined (18 hpi) for VP26 localization by GFP fluorescence and VP1-2 by immunostaining with αVP1-2NT1r. (c) Monolayers of Vero cells were infected with the HSV-1 17 strain harvested at 18 hpi and fractionated as described in Materials and Methods. Total extract (T) and cytoplasmic (C) and nuclear (N) fractions were separated on a 3 to 8% SDS-PAGE gradient gel and subsequently blotted with the specific anti-VP1-2 antibody. Fractionation was assessed using anti-lamin A/C and anti-AP-1 antibodies (lower panel) as markers for nuclear and Golgi compartments, respectively.