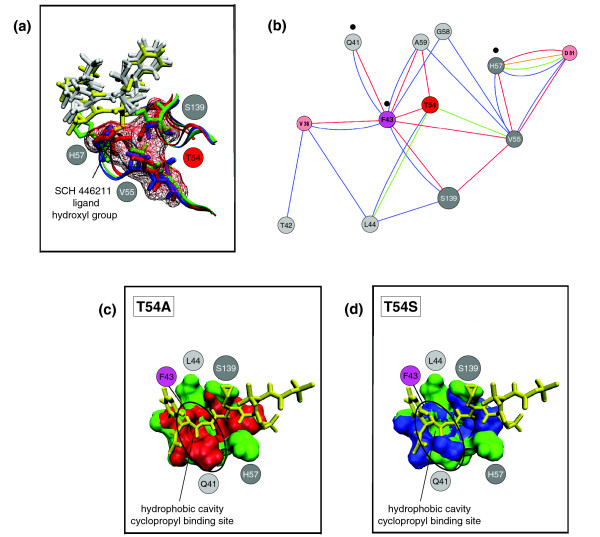
Figure 6.
MD simulations of T54 mutants. (a) Three-dimensional structure analysis of the NS3-4A protease binding pocket taken from the equilibrated structures of the MD simulations of the wild-type (PDB entry 2FM2) and the T54A and T54S mutants (all with ligand SCH 446211). The wild-type protease structure is shown in green and its ligand in yellow. The mutants T54A and T54S are colored red and blue, respectively, and the corresponding ligands white. Part of the surface of the T54A mutant structure is shown in wireframe representation. For the sake of clarity, we have not included the surfaces of the wild-type protein and the T54S mutant structure. The side chains of the residues H57 and S139 in the wild-type structure extend out of the surface of the mutant structure. One of the hydroxyl groups of the SCH 446211 ligand in the wild-type structure clashes with the mutant's surface. Thus, in the mutated structures, this group is rotated by about 90 degrees to the upper right of the inhibitor. (b) Structural changes observed by MD simulations are reflected by the corresponding network of non-covalent residue interactions. Important conformational changes occur at L44 and V55, which do not directly interact with the ligand. Only H57 and S139 contact the ligand, and H57 forms direct interactions with the cyclopropyl group of VX-950. Nodes in the network are highlighted according to the MD simulation results (for details, see legend of Figure 5). (c) Protein surface of the binding pocket for the wild-type protease structure (green) with the ligand SCH 446211 (yellow) in comparison to the T54A mutant structure (red). The changes in the surface (circled in black) correspond to considerable side chain movements as discussed in (a). (d) Protein surface of the binding pocket for the wild-type protease structure (green) with the ligand SCH 446211 (yellow) in comparison to the T54S mutant structure (blue). The changes in the surface area (circled in black) correspond to considerable side chain movements as described in (a).