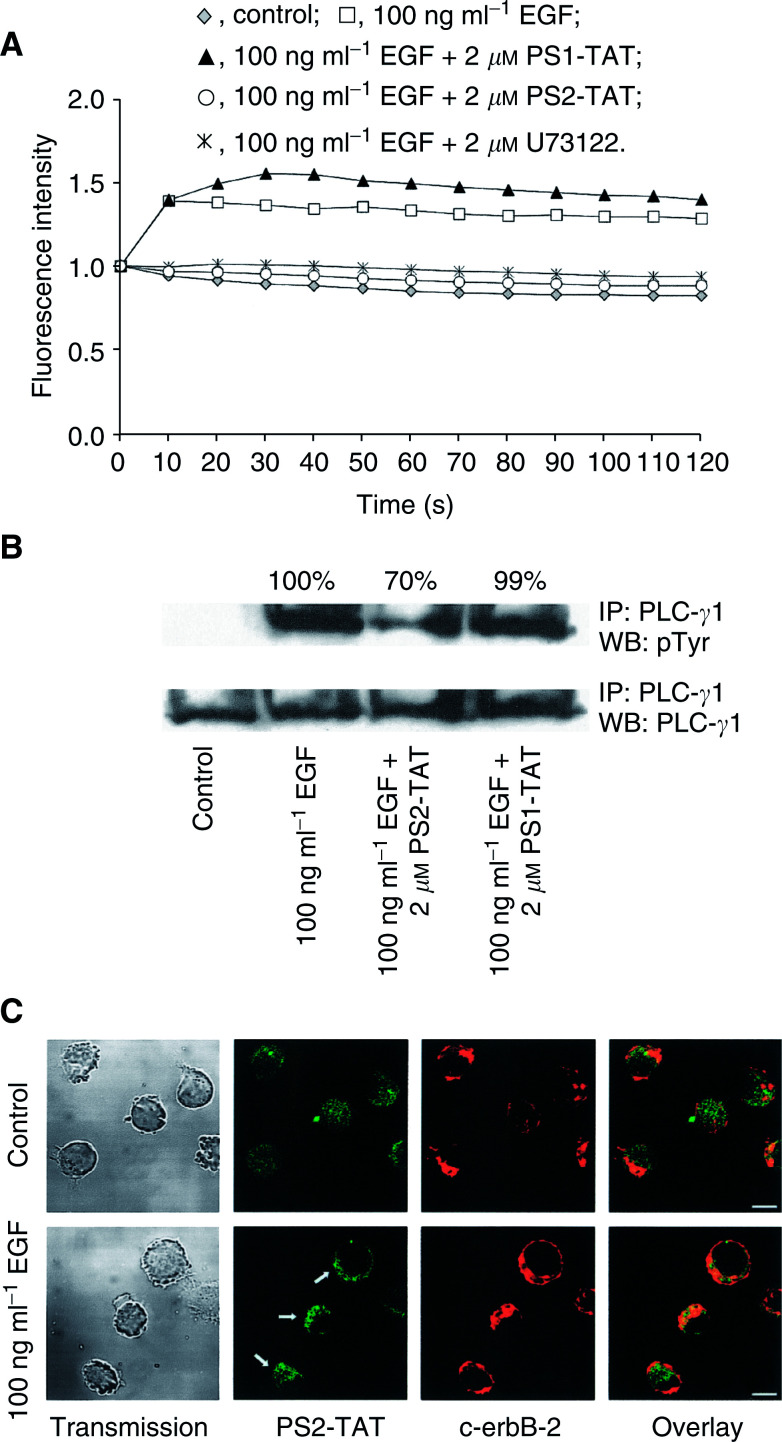
Figure 2.
The EGF-induced PLC-γ1 activation is blocked by PS2-TAT. (A) The EGF-induced calcium influx was used as a read-out to study the inhibitory capacities of the PS1/2-TAT proteins. Treatment of MDA-HER2 cells with PS2-TAT (2 μM) but not PS1-TAT (2 mM) resulted in a complete blockade of the EGF (100 ng ml−1)-induced calcium influx. U73122 (2 μM) was used as a control. (B) Western Blot analysis was performed to study the mode of action as to how PS2-TAT blocks PLC-γ1 activation. Owing to the higher number of cells that were used for these experiments, the amount of PS1/2-TAT was increased accordingly to 133 μM. PS2-TAT treatment resulted in a markedly reduced PLC-γ1 tyrosine phosphorylation in the MDA-HER2 cell line after 2 min of EGF (100 ng ml−1) stimulation. PS1-TAT, did not interfere with PLC-γ1 tyrosine phosphorylation in MDA-HER2 cells. (C) Confocal laser scanning microscope analysis revealed a translocation of PS2-TAT (2 μM) proteins towards the plasma membrane of MDA-HER2 cells after 2 min of EGF (100 ng ml−1) stimulation, where it is found to be colocalised with the constitutively formed EGFR/c-erbB-2 heterodimer (indicated by c-erbB-2 receptor staining). Bar=10 μm.