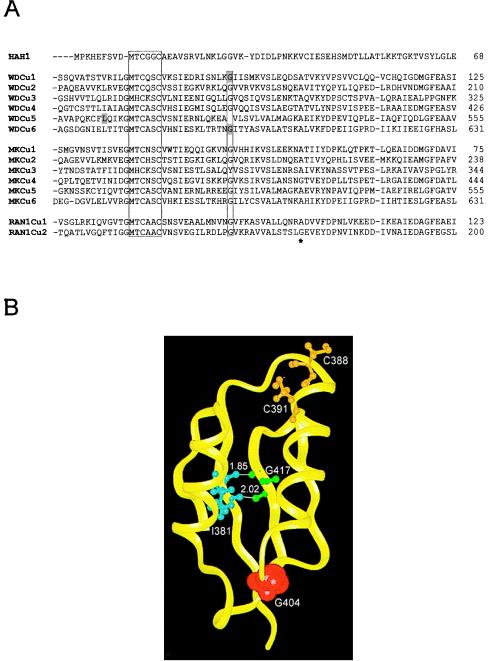
Figure 4.
(A) Amino acid sequence alignment of HAH1 and the copper-binding domains of the Wilson (WD), Menkes (MK), and RAN-1 proteins. The conserved MXCXXC motif and glycine residue are outlined. The Wilson disease mutations (G85V, L492S and G591D) are highlighted, and the RAN-1Cu2 mutation (G173E) noted by asterisk. (B) Structural model (Insight II, Molecular Simulations) of the Menkes fourth copper-binding domain on the basis of the NMR structure (16) depicting the relative positions of the Wilson protein and RAN-1 mutations. C388 and C391 are the copper-binding cysteines. Hydrogen bonds (Å) between I381 and G417 in an antiparallel β-sheet of the Menkes protein are indicated and correspond to L492 in the Wilson protein and G173 in RAN-1, respectively. G404 is shown with van der Waals surface and corresponds to the G85 and G591 Wilson protein mutations.