Abstract
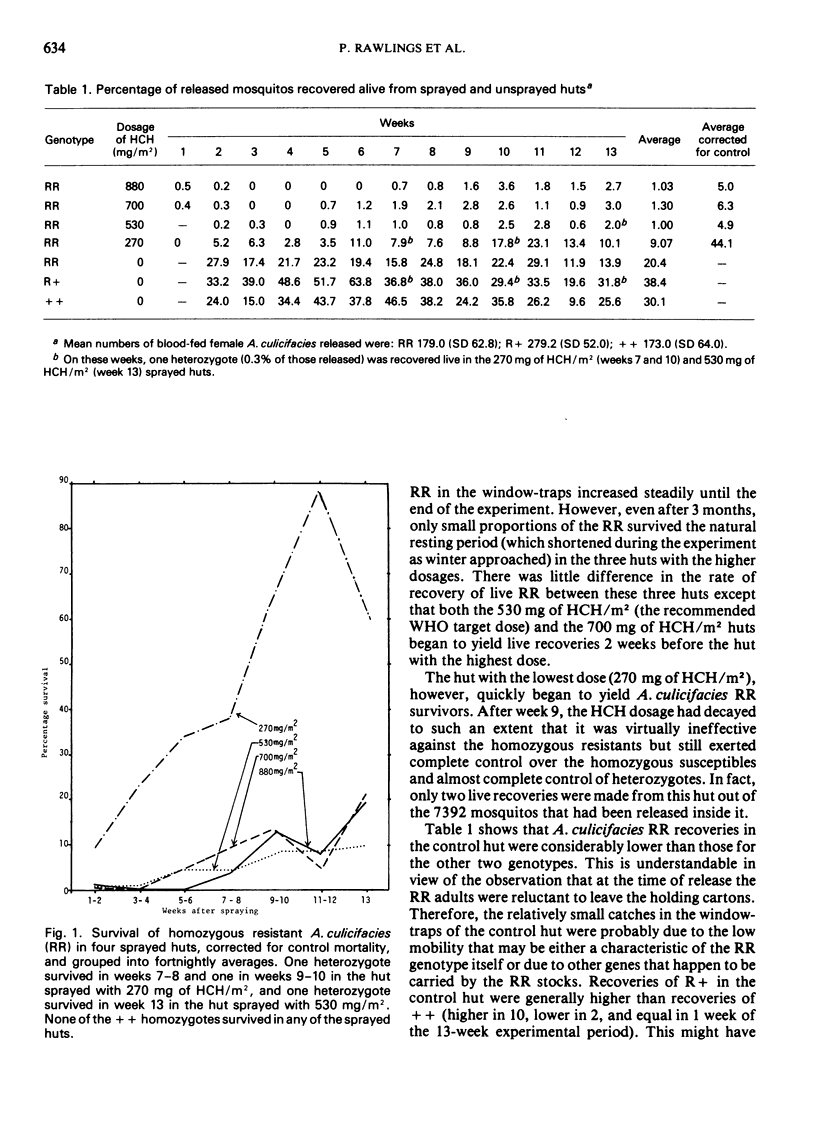
Anopheles culicifacies that were susceptible, heterozygous, or homozygous resistant to HCH and dieldrin were differentially marked with fluorescent dusts and released twice weekly into village huts in Pakistan that had been sprayed with four different dosages of HCH to see which of the genotypes died and which survived. The three highest dosages killed all three genotypes in the first four weeks, and heterozygotes and susceptibles for at least 12 weeks. The lowest dosage killed all the susceptibles throughout the period, and all but 0.07% of the heterzygotes. Thus the resistance is effectively recessive at the higher dosages and unlikely to be selected rapidly, as long as the gene frequency is low to start with and the houses are sprayed regularly. Similar releases of partially and completely resistant A. stephensi, and completely resistant A. subpictus, showed greater survival rates on exposure to the high HCH dosages than the same genotypes of A. culicifacies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comins H. N. The development of insecticide resistance in the presence of migration. J Theor Biol. 1977 Jan 7;64(1):177–197. doi: 10.1016/0022-5193(77)90119-9. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G. DDT-RESISTANCE AND DIELDRIN-RESISTANCE IN ANOPHELES QUADRIMACULATUS. Bull World Health Organ. 1963;29:177–184. [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON G. Insecticide resistance in Anopheles gambiae giles. Nature. 1956 Sep 29;178(4535):705–706. doi: 10.1038/178705a0. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G. Insecticide resistance in Anopheles sundaicus. Nature. 1957 Dec 14;180(4598):1333–1335. doi: 10.1038/1801333a0. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G., POLLARD D. G. Effect of simulated field deposits of gamma-BHC and dieldrin on suceptible, hybrid and resistant strains of Anopheles gambiae Giles. Nature. 1958 Sep 13;182(4637):739–740. doi: 10.1038/182739a0. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G. Studies on insecticide resistance in anopheline mosquitos. Bull World Health Organ. 1958;18(4):579–621. [PMC free article] [PubMed] [Google Scholar]
- Georghiou G. P., Taylor C. E. Genetic and biological influences in the evolution of insecticide resistance. J Econ Entomol. 1977 Jun 15;70(3):319–323. doi: 10.1093/jee/70.3.319. [DOI] [PubMed] [Google Scholar]
- Green C. A., Miles S. J. Chromosomal evidence for sibling species of the malaria vector Anopheles (Cellia) culicifacies Giles. J Trop Med Hyg. 1980 Apr;83(2):75–78. [PubMed] [Google Scholar]
- Reisen W. K. A quantitative mosquito survey of 7 villages in Punjab Province, Pakistan with notes on bionomics, sampling methodology and the effects of insecticides. Southeast Asian J Trop Med Public Health. 1978 Dec;9(4):587–601. [PubMed] [Google Scholar]
- Sakai R. K., Baker R. H., Javed S. Genetic and linkage analyses of dieldrin resistance in Anopheles culicifacies Giles. Trans R Soc Trop Med Hyg. 1979;73(4):445–450. doi: 10.1016/0035-9203(79)90173-1. [DOI] [PubMed] [Google Scholar]


