Abstract
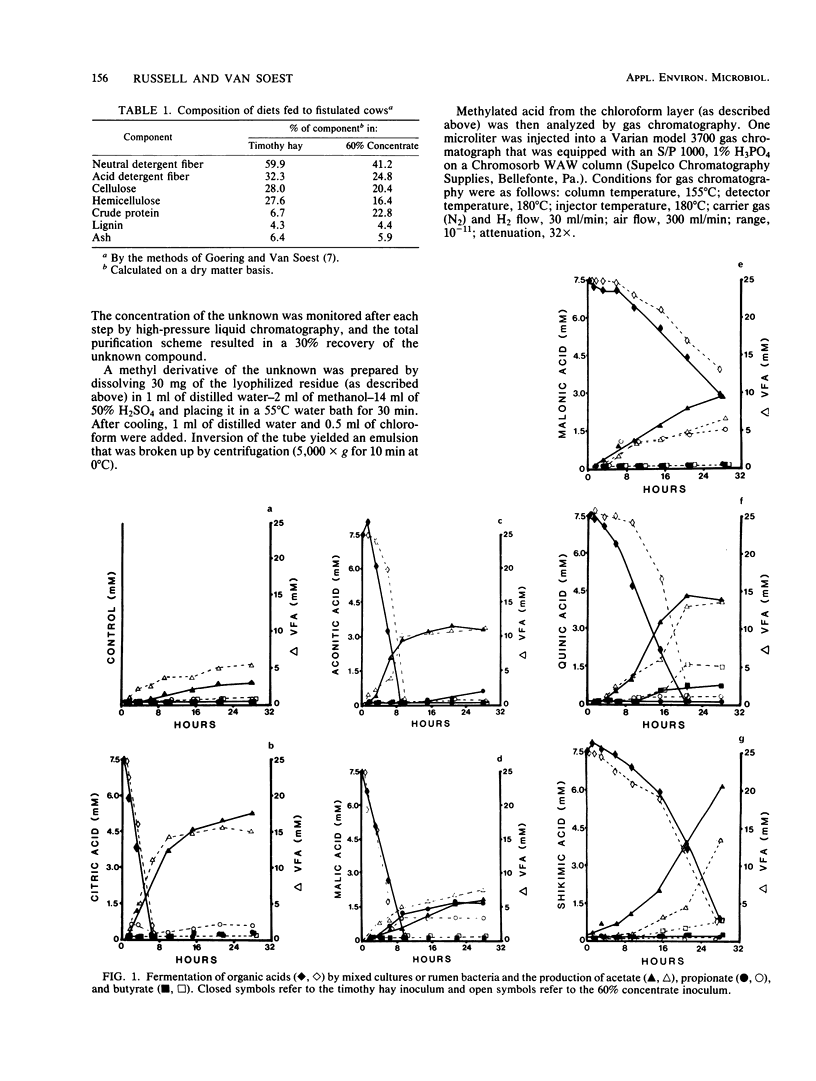
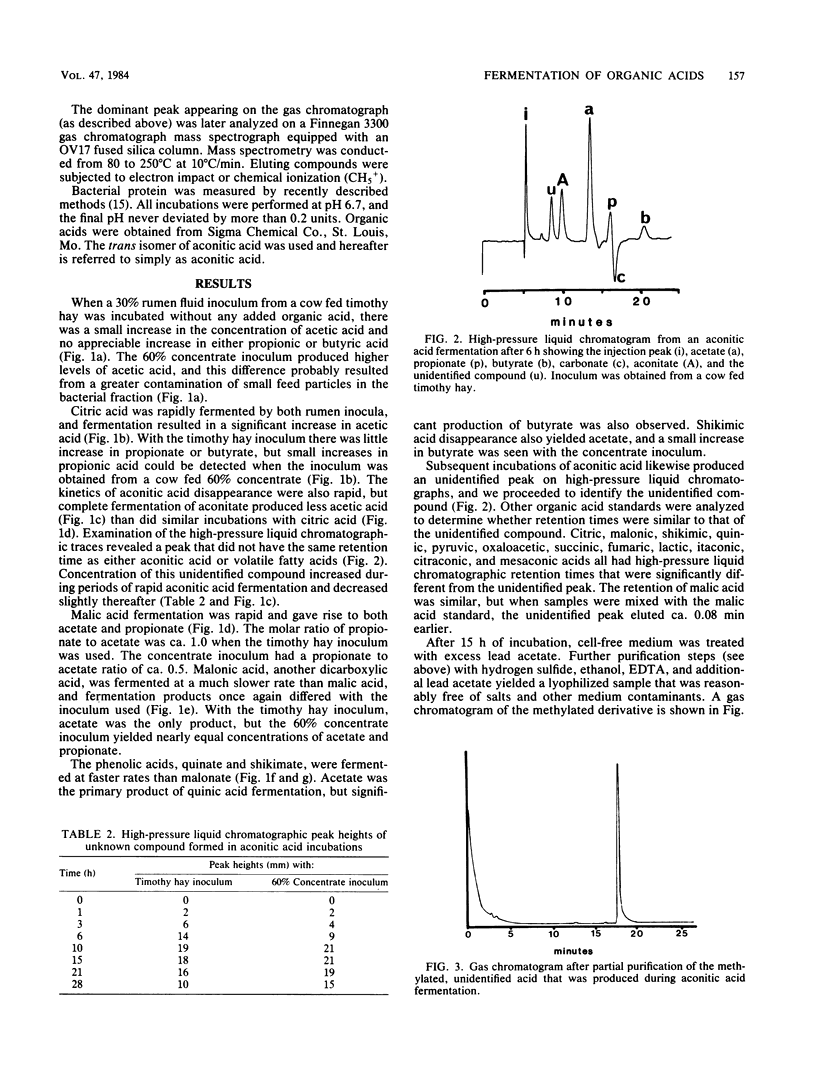
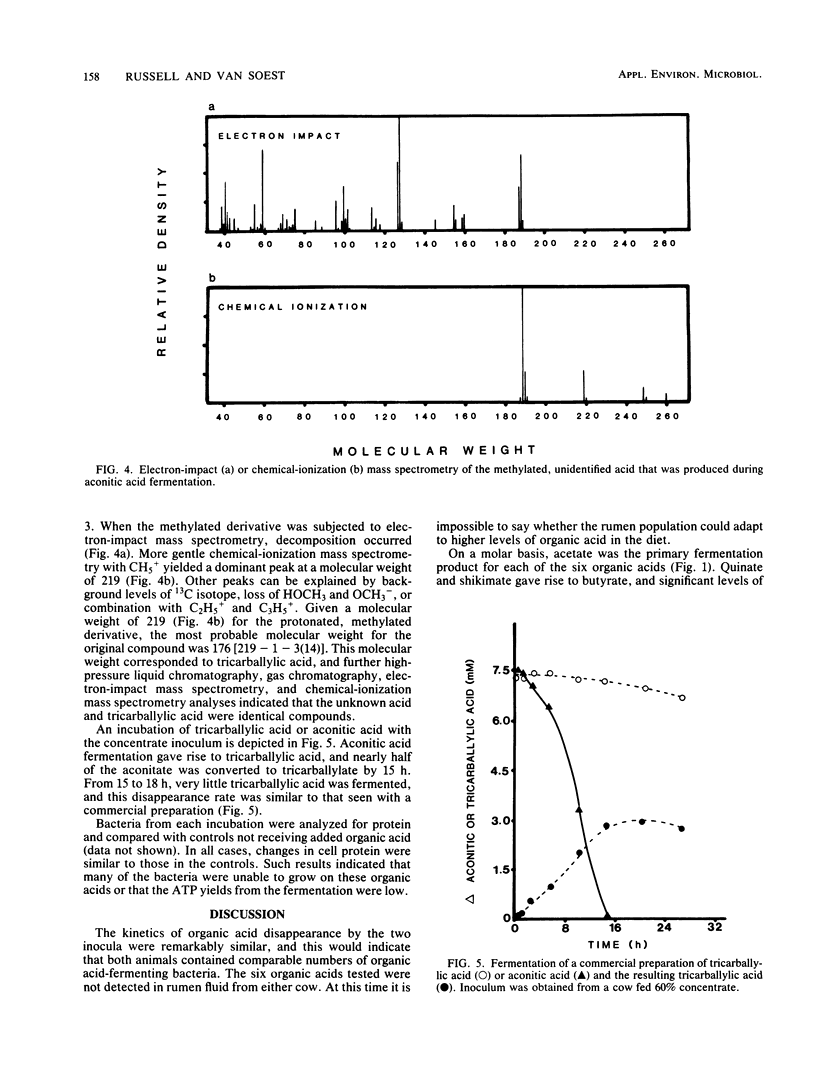
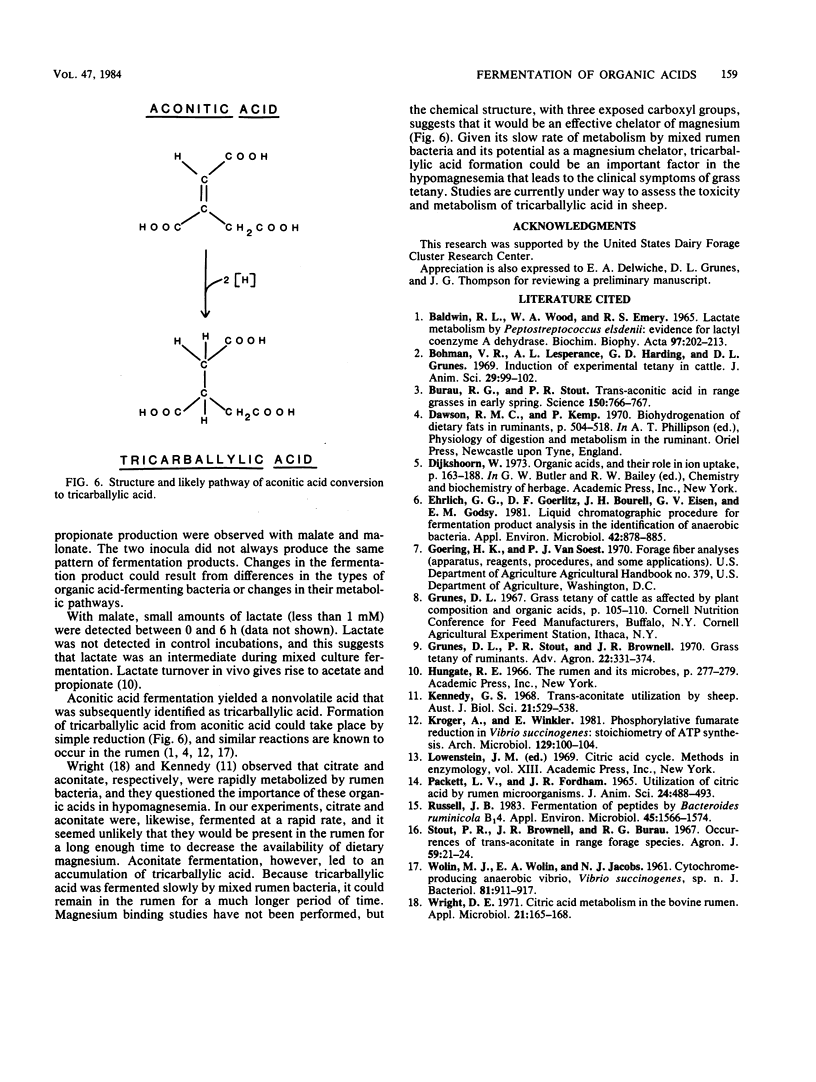
Mixed rumen bacteria from cows fed either timothy hay or a 60% concentrate were incubated with 7.5 mM citrate, trans-aconitate, malate, malonate, quinate, and shikimate. Citrate, trans-aconitate, and malate were fermented at faster rates than malonate, quinate, and shikimate. Acetate was the primary fermentation product for all six acids. Quinate and shikimate fermentations gave rist to butyrate, whereas malate and malonate produced significant amounts of propionic acid. High-pressure liquid chromatography of fermentation products from trans-aconitate incubations revealed a compound that was subsequently identified as tricarballylate. As much as 40% of the trans-aconitate acid was converted to tricarballylate, and tricarballylate was fermented slowly. The slow rate of tricarballylate metabolism by mixed rumen bacteria and its potential as a magnesium chelator suggest that tricarballylate formation could be an important factor in the hypomagnesemia that leads to grass tetany.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- Bohman V. R., Lesperance A. L., Harding G. D., Grunes D. L. Induction of experimental tetany in cattle. J Anim Sci. 1969 Jul;29(1):99–102. doi: 10.2527/jas1969.29199x. [DOI] [PubMed] [Google Scholar]
- Burau R., Stout P. R. Trans-Aconitic Acid in Range Grasses in Early Spring. Science. 1965 Nov 5;150(3697):766–767. doi: 10.1126/science.150.3697.766. [DOI] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G. S. Trans-aconitate utilization by sheep. Aust J Biol Sci. 1968 Jun;21(3):529–538. doi: 10.1071/bi9680529. [DOI] [PubMed] [Google Scholar]
- PACKETT L. V., FORDHAM J. R. UTILIZATION OF CITRIC ACID BY RUMEN MICROORGANISMS. J Anim Sci. 1965 May;24:488–493. doi: 10.2527/jas1965.242488x. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of Peptides by Bacteroides ruminicola B(1)4. Appl Environ Microbiol. 1983 May;45(5):1566–1574. doi: 10.1128/aem.45.5.1566-1574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. E. Citric acid metabolism in the bovine rumen. Appl Microbiol. 1971 Feb;21(2):165–168. doi: 10.1128/am.21.2.165-168.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


