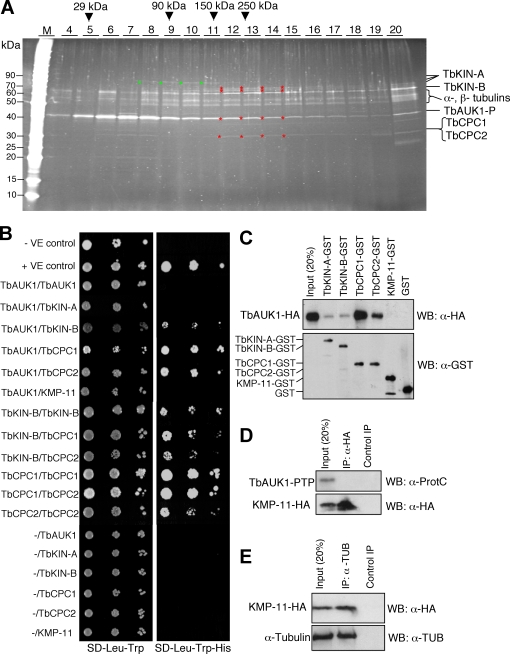
Figure 2. Interactions among TbAUK1 and TbKIN-A, TbKIN-B, TbCPC1 and TbCPC2.
(A). Sucrose density gradient centrifugation of the purified TbAUK1-P complex. Red asterisks indicate the location of a complex among TbAUK1-P and TbKIN-A, TbKIN-B, TbCPC1 and TbCPC2. Yellow asterisks indicate the free forms of TbAUK1-P, TbKIN-B, TbCPC1 and TbCPC2. The green asterisks show the p77 fragment of TbKIN-A. (B). Yeast two-hybrid assay. Full-length TbAUK1, TbKIN-A, TbKIN-B, TbCPC1, TbCPC2 and KMP-11 were each cloned into the pGADT7 vector for expression of proteins fused to the Gal4 activation domain (prey) or into the pGBKT7 vector for expression of proteins fused to the Gal4 binding domain (bait), transformed to yeast strains AH109 and Y187, respectively. Each mated strain was then spotted onto SD-Leu-Trp and SD-His-Leu-Trp plates; with the latter selecting for interacting bait and prey proteins. (C). In vitro GST pull-down monitored on a Western with anti-HA mAb to detect TbAUK1-HA bound to the GST-fusion proteins. Loading of GST-fusion proteins as well as GST alone was monitored by a Western blot with anti-GST mAb. (D). Co-immunoprecipitation testing potential interaction between KMP-11-HA and TbAUK1. (E). Co-immunoprecipitation testing potential interaction between KMP-11-HA and α-tubulin.