Abstract
Previous studies reported that the activity of trigeminal motoneurons innervating masseter muscles is modulated by vestibular inputs. We performed the present study to provide an anatomical substrate for these physiological observations. The transynaptic retrograde tracer pseudorabies virus-Bartha (PRV-BA) was injected into multiple sites of the lower third of the superficial layer of the masseter muscle in rats, a subset of which underwent a sympathectomy prior to virus injections, and the animals were euthanized 24-120 hrs later. Labeled masseteric motoneurons were first found in the ipsilateral trigeminal motor nucleus following a 24-hr post inoculation period; subsequent to 72-hr survival times, the number of infected motoneurons increased, and at ≥ 96-hrs many of these cells showed signs of cytopathic changes. Following 72-hr survival times, a few transynaptically-labeled neurons appeared bilaterally in the medial vestibular nucleus (MVe) and the caudal prepositus hypoglossi (PH) and in the ipsilateral spinal vestibular nucleus (SpVe). At survival times of 96-120 hrs, labeled neurons were consistently observed bilaterally in all vestibular nuclei (VN), although the highest concentration of infected cells was located in the caudal part of the MVe, the SpVe, and the caudal portion of PH. The distribution and density of labeling in the VN and PH were similar in sympathectomized and non-sympathectomized rats. These anatomical data provide the first direct evidence that neurons in the VN and PH project bilaterally to populations of motoneurons innervating the lower third of the superficial layer of the masseter muscle. The MVe, PH, and SpVe appear to play a predominant integrative role in producing vestibulo-trigeminal responses.
Keywords: pseudorabies virus, masseter muscle, vestibular nuclei, prepositus hypoglossi, motor trigeminal nucleus
Introduction
Physiological studies have shown that vestibular inputs modulate the activity of trigeminal motoneurons. In particular, experiments performed on anesthetized guinea pigs (Tolu and Pugliatti 1993; Tolu et al. 1994, 1996; Deriu et al. 1999) revealed that the vestibular system exerts a tonic excitatory influence on the activity of jaw muscles and that stimulation of receptors in the semicircular canal ampullae elicits bilateral excitatory responses of masseter and digastric motoneurons. The functional properties of vestibular-elicited trigeminal responses suggest that multisynaptic pathways connect the vestibular nuclei (VN) with the motor trigeminal nucleus (Mo5) and that the contralateral pathway is more powerful than the ipsilateral one. The same authors have shown that the otolith organ maculae provide bilateral asymmetrical effects on masseter muscles, with the contralateral muscle being more strongly influenced by these signals. The presence of vestibular inputs to trigeminal motoneurons discovered in the animal model has been also demonstrated in humans (Hickenbottom et al. 1985; Deriu et al. 2000). Studies performed on healthy humans described short-duration inhibitory masseter muscle responses elicited bilaterally and symmetrically by vestibular stimulation at latencies consistent with no more than 2-3 synaptic relays (Deriu et al. 2003, 2005).
A number of studies have addressed connections between the VN and the sensory trigeminal complex (Lovick and Wolstencroft 1983; Walberg et al. 1985; Pfaller and Ardvisson 1988; Marfurt and Raichert 1991; Buisseret-Delmas et al. 1999; Valla et al. 2003). Thus far, an anatomical pathway that could transmit to Mo5 signals from the VN or the adjacent prepositus hypoglossi (PH), which also receives direct and multisynaptic inputs from the vestibular endorgans (Kevetter et al 2004; McCrea and Baker 1985; McCrea et al. 1979), has not been clearly defined. Many studies have been performed, using a variety of different methods and animals species, to identify the afferent projections to Mo5 (Vornov and Sutin 1983; Travers and Norgren 1983; Mizuno et al. 1983; Li et al. 1995; Fay and Norgren 1997; Kolta et al. 2000; Shigenaga et al. 2000; Westberg et al. 2001; Inoue et al. 2002). However, none of these studies demonstrated the existence of projections from the VN to trigeminal motoneurons, and only sparse projections from PH to Mo5 were observed. Kolta et al. (Kolta et al. 2000) showed that injections of a retrograde fluorescent tracer into Mo5 resulting in the labeling of VN neurons; however, this finding was dismissed because the investigators surmised that the labeling could have been due to uptake of tracer by fibers of passage as the electrode passed through the cerebellar peduncles and dorso-lateral tegmentum. It is noteworthy, however, that most previous anatomical studies that considered afferents to Mo5 employed monosynaptic tracers that do not cross synapses, whereas physiological studies suggested that a multisynaptic pathway connects the VN with trigeminal motoneurons (Tolu and Pugliatti 1993; Tolu et al. 1994, 1996; Deriu et al. 1999). It is thus not surprising that studies employing monosynaptic tracers failed to reveal the presence of interconnections from the VN or PH to Mo5.
Recently-developed transneuronal tracing techniques employing pseudorabies virus permit the delineation of multisynaptic circuits (Aston Jones and Card 2000; Strick and Card 1992), such as the putative neuronal relays between the VN or PH and Mo5. Fay and Norgren (1997) injected pseudorabies virus into jaw-opening and jaw-closing muscles of bilaterally sympathectomized rats, but did not describe the presence of infected neurons in the VN or PH subsequent to these injections. However, the locations of their injection sites into the jaw muscles were not discussed in detail, and these mandible muscles are known to have a complex architecture. For example, the masseter muscle is multipennate (Eriksson 1982; Eriksson and Thornell 1983; Stålberg et al. 1986), and is comprised of several functionally-distinct compartments that differ with respect to morphological and biochemical properties (Herring et al. 1979; Widmer et al. 2003). Vestibular stimulation differentially affected the firing of masseter muscle compartments (Deriu et al. 2003, 2005); vestibular-elicited responses were largest in magnitude in the compartment receiving the heaviest innervation by motoneurons, which is localized in the lower third of the superficial layer of the muscle (Godeaux and Desmedt 1975; Widmer and Lund 1989; Widmer et al. 1997). Given this, it is possible that the lack of VN or PH labeling in Fay and Norgren’s (1997) study was due to the fact that their pseudorabies virus injections did not include this region.
In the present study, pseudorabies virus was specifically injected into the lower third of the superficial layer of the masseter muscle, and animals were euthanized at several time points to delineate pathways providing potentially direct and multisynaptic influences on this muscle’s activity. We tested the hypothesis that a polysynaptic pathway connects the VN and PH with masseter motoneurons.
Methods and materials
All procedures used in this study were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were conducted on 26 adult male rats (Sprague Dawley, 250-350 g) purchased from Charles River Laboratories (Wilmington, MA, USA). Throughout the course of the study, animals were maintained under Biosafety level 2 conditions, as defined by U.S. Department of Health and Human Services publication number CDC 88-8395.
The Bartha strain of pseudorabies virus (PRV-BA) was employed for transneuronal tracing; the characteristics of this virus were described previously (Bartha 1961; Card et al. 1992; Yang et al. 1999). The virus was the generous gift of Dr. Lynn Enquist (Princeton University, Princeton, NJ, USA). PRV-BA was grown in pig kidney (PK15) cells, adjusted to a final concentration of 1.7 × 109 pfu/ml, cleared of cellular debris by centrifugation, aliquoted at 40 μl per tube, and stored at -80°C. Individual aliquots of virus were thawed immediately prior to the injection. Excess virus was inactivated with bleach and discarded.
Surgical procedures
All surgical procedures were performed using aseptic techniques. Anesthesia was induced in one group of 16 animals using 5% isoflurane vaporized in O2 and administrated through a facemask; subsequently, a 0.5-2% concentration was employed to maintain areflexia. An incision was made under the chin to access the lower third of the masseter muscle, and a total volume of 40 μl of PRV-BA was injected at multiple sites in the superficial layer of the muscle using a 10 μl Hamilton syringe. To reduce the possibility of virus spread, the needle was left in place for 1 min after each injection, and the area was subsequently swabbed with saline. After the injections were completed, the skin was sutured closed.
In the other 10 animals, a unilateral sympathectomy was performed prior to injecting PRV-BA into the masseter muscle on the same side. This procedure was executed to eliminate the sympathetic nervous system innervation in the target muscle, which also could have transported virus to the central nervous system. This second group of animals was anesthetized using a mixture of ketamine (50 mg/kg), xylazine (6 mg/kg) and acepromazine (0.5 mg/kg) injected i.m. To perform the sympathectomy, the midline neck was incised, and the sternohyoid muscle was gently retracted in order to access the common carotid artery next to the trachea. The artery was separated from the surrounding connective tissue in order to expose the sympathetic nerve, which was sectioned just distal to the carotid sinus. Subsequently, PRV-BA was injected into the masseter muscle as described above. One animal in this group was used as a control to determine if PRV-BA could have spread to non-target tissues in these experiments. In this case, the masseteric nerve was also sectioned.
Ketoprofen (5 mg/kg) was injected intramuscularly to provide analgesia; additional injections were made at 12-hr intervals if any indications of pain and distress were present. After recovery from anesthesia, animals were returned to their home cage for the balance of the survival period. The 16 animals without a sympathectomy were sacrificed subsequent to the following survival times: 24-hrs (n=2), 48-hrs (n=4), 72-hrs (n= 4), 84-hrs (n=4), or 96-hrs (n=2). The 10 animals that underwent a sympathectomy were sacrificed after 72-hrs (n=4), 96-hrs (n=3), or 120-hrs (n=3). Included in the latter group of sympathectomized animals was the control rat whose masseteric nerve was also sectioned, which survived for 72 hrs after the virus injection.
Tissue processing
After their respective survival times, the animals were anesthetized using an overdose of sodium pentobarbital (100 mg/kg injected i.p.), and perfused transcardially using 300 ml of saline followed by 300 ml of a 4% paraformaldehyde fixative solution (McLean and Nakane 1974). Subsequently, the brainstem and thoracic spinal cord segments were removed, stored in fixative at 4°C for 1 day, and transferred to a 30% phosphate-buffered sucrose solution at 4°C for two days. Transverse sections of the pons and medulla were cut at 30 μm with a freezing microtome, and thoracic cord segments were cut either horizontally or transversally at 30 μm. Tissue was collected in six sequential wells, such that each well contained a rostrocaudal series of sections spaced 180 μm apart, and stored at -20°C in cryoprotectant (de Olmos et al. 1978) until processed for immunohistochemical localization of PRV-BA infected neurons.
All wells of brainstem sections and one bin of thoracic cord sections were incubated in a solution containing rabbit polyclonal antiserum raised against acetone-inactivated PRV-BA (Rb-133, 1:10,000, obtained from Dr. Lynn Enquist) for 2 days. Subsequently, tissue was processed using the avidin-biotin modification of the peroxidase-antiperoxidase procedure (Hsu et al. 1981), which employed affinity purified donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Vectastain Elite avidin-biotin reagents (Vector Laboratories, Burlingame, CA, USA). Upon completion of the immunohistochemical processing, the tissue was mounted on gelatin coated slides, counterstained with methyl green, dehydrated, cleared, and coverslipped with Cytoseal 60 (VWR Scientific, West Chester, PA, USA).
Tissue analysis
Tissue sections were examined using an Olympus BH-2 microscope to determine the distribution of labeled neurons in thoracic spinal cord and brainstem, particularly in the Mo5, VN, and PH. The locations of infected neurons in the latter structures were plotted on templates spaced ∼200 μm apart generated mainly with reference to Rubertone’s templates (Rubertone et al. 1995). During analyses, the caudal prepositus hypoglossi (CPH), defined as the portion of the nucleus at the level of or caudal to the external cuneate nucleus, was considered to be a separate region from the rostral part of the nucleus (RPH). Digitized photographs were obtained with the use of a Nikon Eclipse E600 photomicroscope equipped with an RT Monochrome digital camera (Diagnostic Instruments Inc, Sterling Heights, MI, USA) and the MetaMorph version 6.1r4 image analysis system (Universal Imaging Corporation, Downingtown, PA, USA). Digital images were prepared for publication using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA, USA). Individual images were adjusted for size, brightness and contrast.
Results
No brainstem labeling was observed in the control animal in which a sympathectomy was performed and the masseteric nerve was transected, indicating that virus had not spread from the masseter muscle to adjacent muscles with intact motor innervation. In contrast, brainstem labeling was observed in all other rats. Moreover, infected neurons were observed in the VN of all animals euthanized following a survival time of 72 hours or longer, with the exception of an animal that survived 96 hrs (that had not received a sympathectomy) and a sympathectomized animal that survived 120 hrs subsequent to virus injections. These animals were not included in our analyses.
Non-sympathectomized rats surviving ≥ 72 hrs exhibited infection of neurons located in the intermediolateral (IML) cell column in the thoracic spinal cord. The number of infected IML neurons increased with longer survival times, although the viral antigen remained confined to the soma and proximal dendrites of the infected cells. Infected IML neurons were absent in sympathectomized animals, with the exception of 2 animals that survived 120 hrs following the PRV injections into the masseter muscle. In one animal, only a few labeled neurons were detected in the IML, although a substantial IML infection was observed in the other case. It is noteworthy that this was also the animal surviving 120 hrs after virus injections into the masseter that exhibited little labeling in the VN or PH. This observation suggests that over the time course of our experiment, it is unlikely that the labeling of vestibular nucleus neurons was due to the transneuronal passage of virus through the sympathetic nervous system. Furthermore, no differences in the number or distribution of infected VN or PH neurons could be distinguished in sympathectomized and non-sympathectomized animals, and thus the findings from the two groups were pooled.
Labeling in the trigeminal motor nucleus
Following a 24-hr survival time subsequent to PRV-BA injections into the masseter muscle, a very few labeled motoneurons (∼2-5 cells per each well of tissue containing one-sixth of the brainstem sections for an animal) could be detected in the ipsilateral Mo5. Survival times of 48 hrs resulted in a larger number of infected trigeminal motoneurons (∼9-12 cells/well), particularly in the lateral portion of Mo5. After survival times of 72 and 84 hrs, labeled motoneurons were consistently observed in the ventral and dorsolateral portions of the ipsilateral Mo5, and their number averaged ∼22 cells/well. The distribution of infected motoneurons differed in the rostral and caudal portions of Mo5. In the caudal regions of the nucleus, labeled motoneurons were observed ventrally, whereas they were mostly found in the lateral and dorsolateral aspects of Mo5 at its rostral level (Fig. 1). In some cases, a few labeled motoneurons were also observed in the medial and central portions of the motor nucleus.
Figure 1.
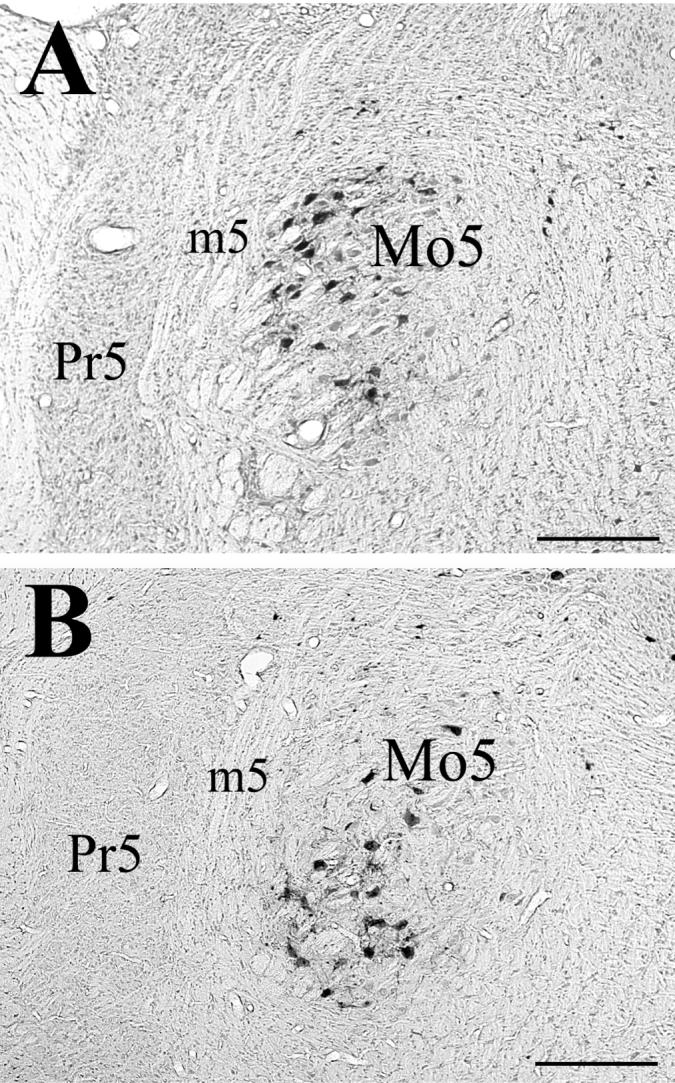
Photomicrographs of labeled motoneurons in the (A) rostral and (B) caudal portions of the trigeminal motor nucleus, from an animal that survived 72 hrs following PRV-BA injections into ipsilateral masseter muscle. Abbreviations: m5, motor root of 5th nerve; Mo5, trigeminal motor nucleus; Pr5, principal sensory nucleus. Scale bars represent 500 μm.
Subsequent to a 96-hr postinoculation survival time, the number of labeled motoneurons in the ipsilateral Mo5 was larger than noted after shorter survival periods: an average of ∼40 cells/well. However, the distribution of the motoneurons in Mo5 was similar to that observed at earlier survival times (72-84 hrs). By the 96-hr survival time, many motoneurons showed signs of cytopathic changes in their morphology and, in addition, a few infected motoneurons could be detected in the contralateral Mo5 (∼4 motoneurons/well). In animals surviving 120 hrs after the virus injections, fewer motoneurons were labeled (∼10 cells/well) than following shorter survival periods, and those cells showed significant cytopathic changes, presumably due to the effects of the protracted viral infection. The reduction in numbers of labeled motoneurons was probably because most of the longest-infected cells had degenerated.
Labeling in the vestibular nuclei and nucleus prepositus hypoglossi
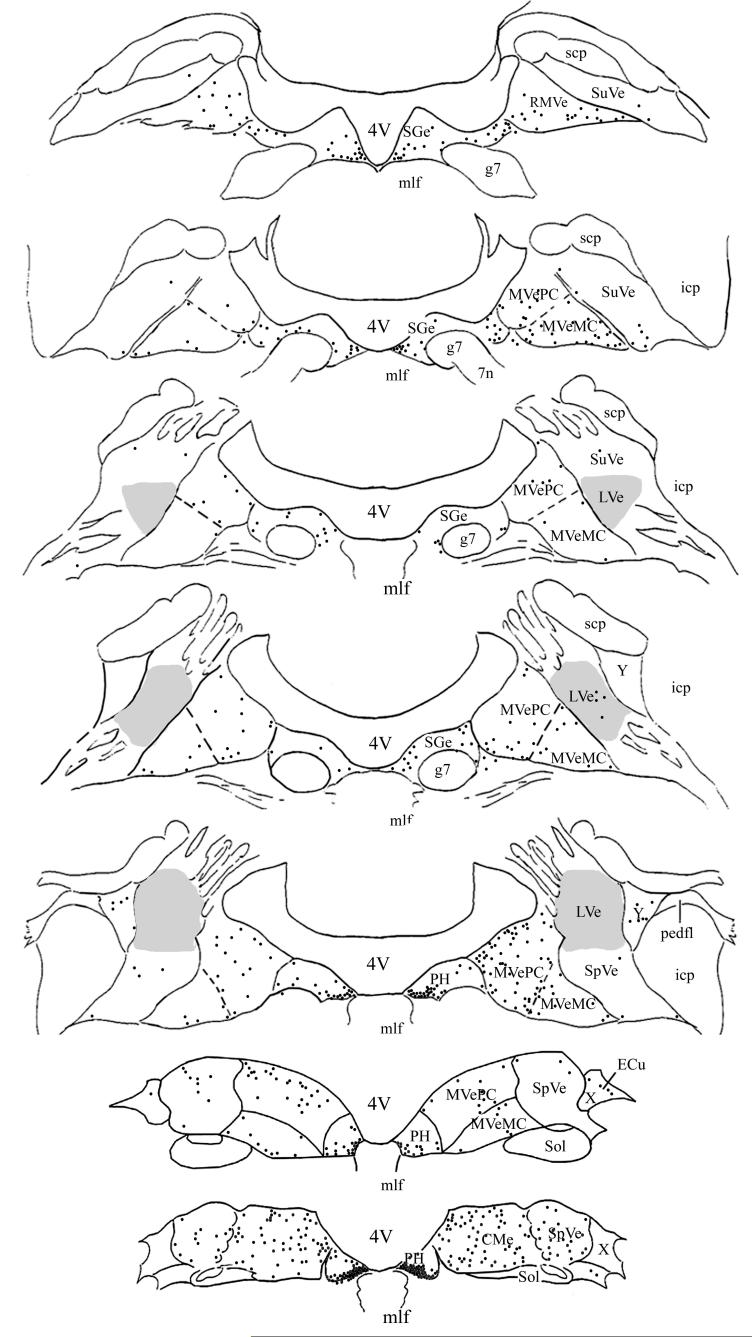
PRV-BA injections into the left masseter muscle resulted in a bilateral infection of VN neurons following survival times as short as 72 hrs. At this survival time, the labeled neurons were distributed bilaterally but sparsely in the caudal PH, along the entire extent of the MVe, and in the ipsilateral spinal vestibular nucleus (SpVe), as indicated in Table 1. Following an 84-hr survival time, labeling increased slightly in these regions, and also appeared in all the other VN (see Table 1). In one animal (case 2), labeling in the caudal portion of PH was especially dense, where the infected cells were concentrated in the ventromedial part of the nucleus on both the ipsilateral and contralateral sides.
Table 1.
Number of neurons in different regions of the vestibular nuclei (VN) and the caudal (CPH) and rostral (RPH) portions prepositus hypoglossi (PH) that were infected 72 or 84 hours following injections of PRV-BA into the masseter muscle. Labeling is depicted for each animal that exhibited infected VN neurons (7 animals that survived for 72 hours and 4 animals that survived for 84 hours). Each row indicates cell counts for a particular animal; labeling ipsilateral and contralateral to the side injected with virus is shown in adjacent rows. Abbreviations are the same as in Fig. 2, with the following additions: Ipsi, ipsilateral; Contra, contralateral
| Case # | Side | Region of VN Complex and PH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPH | RPH | MVePC | MVeMC | SpVe | CMVe | RMVe | SuVe | LVe | X | Y | ||
| 72-hour survival time | ||||||||||||
| 1 | Ipsi | 1 | - | - | - | 2 | 1 | 2 | - | - | - | - |
| Contra | - | - | - | - | - | - | 1 | - | - | - | - | |
| 2 | Ipsi | 7 | - | - | - | 2 | 6 | - | - | - | - | - |
| Contra | 1 | - | - | - | - | 6 | - | - | - | - | - | |
| 3 | Ipsi | - | - | - | - | - | - | - | - | - | - | - |
| Contra | - | - | 1 | - | - | - | - | - | - | - | 1 | |
| 4 | Ipsi | - | - | - | - | - | - | - | - | - | - | - |
| Contra | - | - | - | - | - | - | 1 | - | - | - | - | |
| 5 | Ipsi | - | - | - | - | - | - | 3 | - | - | - | - |
| Contra | - | - | 1 | - | - | - | 1 | - | - | - | - | |
| 6 | Ipsi | - | - | 7 | - | - | 3 | 1 | - | - | - | - |
| Contra | 3 | - | 3 | - | - | 1 | 1 | - | - | - | - | |
| 7 | Ipsi | 1 | - | - | - | - | 3 | - | - | - | - | - |
| Contra | - | - | - | - | - | 1 | - | - | - | - | - | |
| 84-hour survival time | ||||||||||||
| 1 | Ipsi | 6 | - | 5 | 1 | 3 | 3 | - | - | 1 | - | 1 |
| Contra | 6 | - | 4 | - | 1 | - | 1 | - | - | - | - | |
| 2 | Ipsi | 97 | - | 6 | - | 4 | 9 | 6 | - | 2 | - | 1 |
| Contra | 88 | - | 11 | - | 18 | 12 | 4 | 1 | - | - | - | |
| 3 | Ipsi | 11 | - | - | - | 1 | 2 | 2 | - | - | - | - |
| Contra | 11 | - | 1 | 1 | 1 | 1 | - | 1 | - | - | - | |
| 4 | Ipsi | 7 | - | 1 | 2 | 1 | 1 | 2 | - | - | - | - |
| Contra | 2 | - | - | - | - | 1 | - | - | - | - | - | |
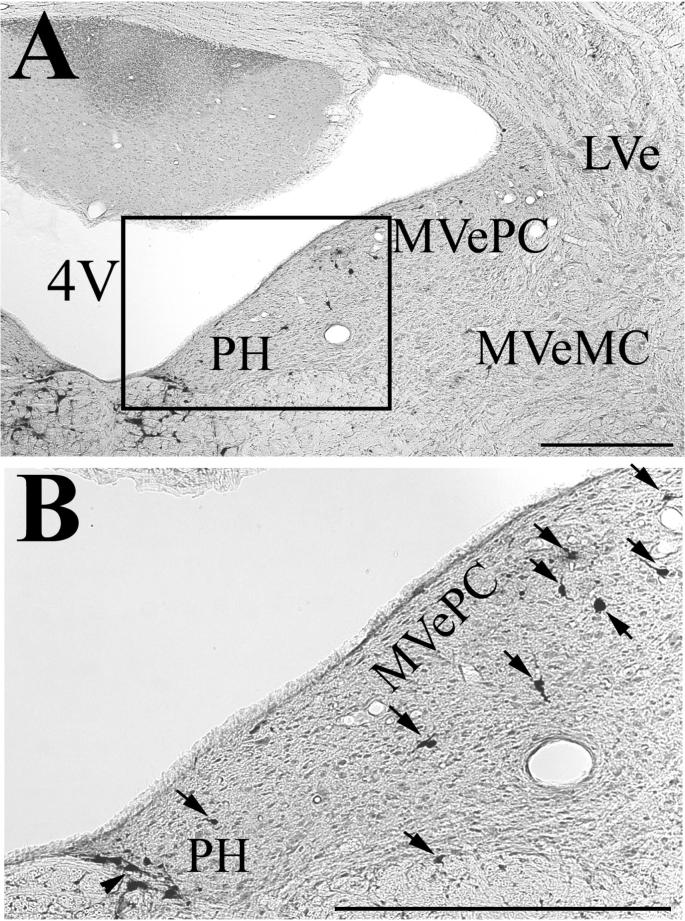
Following a 96-hr postinoculation period, the number of infected cells increased considerably in both the ipsilateral and contralateral VN, particularly in the caudal portion of the MVe, the SpVe, and the caudal PH, as shown in Table 2. Within PH, most infected neurons were concentrated in the ventromedial aspect of the nucleus, although some labeled cells were located dorsally. Fig. 2 shows the distribution of labeled cells on templates of the VN and PH for one animal surviving 96 hrs following the virus injections. Photomicrographs illustrating transynaptically-labeled neurons in the contralateral VN and PH of an animal euthanized following a 96-hr survival period are provided in Fig. 3. At 120 hrs following the PRV-BA injections, many cells in the areas described above showed cytopathic changes, but the labeling pattern was similar to that following 96-hr survival times, as indicated in Table 2.
Table 2.
Number of neurons in different regions of the VN and PH that were infected 96 or 120 hours following injections of PRV-BA into the masseter muscle. Labeling is depicted for each animal that exhibited infected VN neurons. The organization of this table is similar to that of Table 1
| Case # | Side | Region of VN Complex and PH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPH | RPH | MVePC | MVeMC | SpVe | CMVe | RMVe | SuVe | LVe | X | Y | ||
| 96-hour survival time | ||||||||||||
| 1 | Ipsi | 136 | 3 | 16 | 29 | 109 | 130 | 10 | 1 | 12 | 2 | |
| Contra | 158 | 1 | 28 | 16 | 122 | 158 | 14 | 25 | 1 | 2 | 2 | |
| 2 | Ipsi | 235 | 45 | 62 | 26 | 144 | 248 | 13 | 5 | 1 | - | 5 |
| Contra | 296 | 39 | 83 | 49 | 172 | 259 | 18 | 11 | 4 | 4 | 6 | |
| 3 | Ipsi | 60 | 6 | 11 | 3 | 22 | 40 | 5 | 4 | 2 | - | 2 |
| Contra | 57 | 13 | 12 | 5 | 25 | 46 | 12 | 5 | 1 | - | - | |
| 4 | Ipsi | 85 | 25 | 78 | 37 | 66 | 122 | 14 | 10 | 2 | 2 | 2 |
| Contra | 108 | 30 | 71 | 34 | 87 | 153 | 14 | 9 | 1 | 4 | 2 | |
| 120-hour survival time | ||||||||||||
| 1 | Ipsi | 202 | 6 | 24 | 22 | 78 | 53 | 6 | 10 | 2 | - | 3 |
| Contra | 213 | 14 | 20 | 9 | 74 | 73 | 5 | 7 | 1 | 1 | 3 | |
| 2 | Ipsi | 114 | 6 | 24 | 9 | 26 | 55 | 2 | - | 1 | 3 | 5 |
| Contra | 123 | 6 | 31 | 19 | 42 | 77 | - | 5 | 2 | 2 | - | |
Figure 2.
Distribution of PRV-BA-immunopositive neurons in the vestibular nuclei and prepositus hypoglossi of one animal that survived 96 hrs following virus injections into the masseter muscle. The left side of the diagram depicts labeling that was ipsilateral to the injection site, whereas the right side shows contralateral labeling. The locations of transneuronally-labeled neurons were plotted on 7 representative sections, which were derived from Rubertone’s templates (Rubertone et al. 1995). Each black dot represents one infected cell. A shaded area demarks the lateral vestibular nucleus (LVe). Abbreviations: 4V, Fourth ventricle; 7n, root of facial nerve; CMVe, caudal portion of the medial vestibular nucleus; ECu, external cuneate nucleus; g7, genu of facial nerve; icp, inferior cerebellar peduncle; mlf, medial longitudinal fasciculus; MVeMC, magnocellular portion of the medial vestibular nucleus; MVePC, parvocellular portion of the medial vestibular nucleus; pedfl, floccular peduncle; PH, prepositus hypoglossi; RMVe, rostral portion of medial vestibular nucleus; scp, superior cerebellar peduncle; Sge, supragenual nucleus; Sol, nucleus of the solitary tract; SpVe, spinal vestibular nucleus, SuVe, superior vestibular nucleus; X, X-group; Y, Y-group.
Figure 3.
Photomicrographs showing examples of infected neurons in the parvocellular (MVePC) and magnocellular (MVeMC) portions of the contralateral medial vestibular nucleus and nucleus prepositus hypoglossi (PH), from an animal euthanized 96 hrs following PRV-BA injections into the masseter muscle. The area demarked by a box in panel A is shown at higher magnification in panel B. Infected cells are indicated in panel B by arrows; typically an arrow demarks one cell, but labeling was so dense in the ventromedial PH that one arrow identifies a cluster of infected neurons. Abbreviations: 4V, fourth ventricle; LVe, lateral vestibular nucleus. The scale bar represents 500 μm.
It was incidentally noted that the largest number of labeled neurons was present in the VN, particularly MVe, and in PH when a substantial number of infected motoneurons was present in the lateral part of the Mo5. For example, in the two animals in which VN labeling was nearly absent (surviving 96 and 120 hours after the PRV injections into the masseter muscle), the lateral region of Mo5 was devoid of labeling, although labeling was dense in other portions of the nucleus.
Distribution of labeled neurons in other regions of the brainstem
Injections of PRV-BA into the masseter muscle also produced neuronal labeling in regions of the brainstem other than the Mo5, VN, and PH. As early as 48-hr survival times, a limited number of labeled cells could be observed in a variety of subdivisions of the reticular formation, including the intermediate reticular nucleus, the parvocellular reticular formation, and portions of the gigantocellular nuclei. A small number of infected cells was also present in the locus coeruleus and subcoeruleus alpha nucleus, nucleus raphe obscurus and magnus, subdivisions of the spinal trigeminal complex, and the nucleus of the solitary tract. At 72-hr survival times, the number of infected neurons increased in these regions, and labeling also appeared in other subdivisions of the reticular formation, including: the lateral reticular nucleus, the lateral paragigantocellular nucleus, the caudo-ventral reticular nucleus, the rostro-ventro-lateral nucleus, and the pontine reticular nucleus. This labeling was bilateral, with an ipsilateral predominance. A few infected cells also appeared in the supratrigeminal nucleus, the intertrigeminal nucleus, the principal trigeminal sensory nucleus, the inferior olive, the accessory trigeminal nucleus, the supragenual nucleus, and the periaqueductal gray. Labeled neurons were also occasionally observed in the mesencephalic trigeminal nucleus (Mes5). Subsequent to longer survival times, the number of infected neurons increased considerably in all the brain regions described above, except Mes5, and additionally appeared in the caudal interstitial nucleus of the medial longitudinal fasciculus.
Discussion
This study demonstrated that a neural pathway is present to relay signals from the VN and PH to masseter motoneurons. This observation provides an anatomical substrate to account for the finding that vestibular stimulation alters masseter muscle activity in animals (Tolu and Pugliatti 1993; Tolu et al. 1994, 1996; Deriu et al. 1999) and humans (Hickenbottom et al. 1985; Deriu et al. 2000, 2003, 2005). All parts of the VN contained infected neurons following injections of PRV-BA into the masseter muscle, especially the caudal portion of the MVe and the SpVe; in addition, labeling was concentrated in the caudal PH. These latter regions apparently play a predominant role in mediating vestibular influences on masseter muscle activity.
Several pieces of evidence suggest that the VN and PH labeling resulting from injection of PRV-BA into the masseter muscle was due to passage of virus through specific motor circuitry regulating the activity of this muscle. Although the peripheral processes of afferent fibers can uptake PRV and transport the virus retrogradely to cell bodies in ganglia (Card 1998; Aston-Jones and Card 2000), in rodents PRV is not transported anterogradely from ganglion cell bodies along the central processes of the afferents into the central nervous system (CNS)(Hofstetter et al. 2005). Accordingly, we did not observe any evidence of the infection of trigeminal afferents within the brainstem. Furthermore, within the CNS, PRV is transmitted almost exclusively in the retrograde direction, as the receptors for the virus are mainly confined to axon terminals (Strick and Card 1992; Card 1998; Aston-Jones and Card 2000). This evidence suggests that infection of brainstem neurons occurred via retrograde passage of PRV through motor circuitry instead of via anterograde transmission of virus through sensory pathways.
The distribution and number of infected VN and PH neurons was similar in sympathectomized animals and those without a sympathectomy, suggesting that the transneuronal passage of virus to these regions did not occur through autonomic circuitry. In addition, the positions of masseter motoneurons within Mo5 observed in our experiments were similar to those noted in previous studies (Mizuno et al. 1975; Weijs 1996; Fay and Norgren 1997), indicating that PRV-BA had not been transported by efferents innervating other muscles. Furthermore, the locations of infected medullary neurons outside of the VN and PH were similar to those reported in a manuscript by Fay and Norgren (Fay and Norgren 1997), who also injected PRV-BA into the masseter muscle. Curiously, however, this latter study did not describe the presence of labeling within the VN complex and PH. The basis of this difference between their findings and ours is not clear, but one possibility is that the discrepancy is related to the placement of injections within the masseter muscle. To our knowledge, no data are available regarding the organization of masseter compartments and their innervation in rats, but this information is available for other species, including the rabbit (Widmer et al. 1997), pig (Herring et al. 1979) and human (Godeaux and Desmedt 1975; Stålberg et al. 1986; Widmer and Lund 1989). In these species, the masseter muscle has been shown to be composed of multiple anatomical partitions that each produce different mechanical actions (Herring et al. 1979; Widmer et al. 2003). Moreover, the muscle fibers associated with each masseter motor unit are limited to a relatively small region of the muscle, such that each masseter motoneuron provides innervation to a restricted territory (Weijs et al. 1993). Our PRV-BA injections were made into the lower third of the superficial layer of the muscle, because this compartment receives the densest motor innervation (Godeaux and Desmedt 1975; Widmer and Lund 1989; Widmer et al. 1997); in humans, the largest vestibular-induced EMG responses were detected when recording electrodes were positioned on this part of the muscle (Deriu et al. 2003, 2005). The compartments of the masseter muscle injected with PRV-BA in Fay and Norgren’s study (Fay and Norgren 1997) were not discussed, such that the locations of their injections within the muscle could have differed from ours. It is possible that the vestibular system influences are strongest in the middle part of the lower third of the superficial layer of the masseter muscle, and that injections of virus elsewhere in the muscle produce little or no infection of VN or PH neurons. Further experiments will be required to determine whether there is topography in vestibular system influences on the compartments of the masseter muscle.
The earliest infection of VN and PH neurons occurred following a postinoculation transport time of 72 hrs, but substantial labeling in these regions occurred only after 96 hrs. In contrast, the earliest infection of cells in regions of the reticular formation adjacent to the VN and in the spinal trigeminal nucleus occurred within 48 hrs. Labeling was observed in other brainstem structures, including the principal trigeminal sensory nucleus and the reticular zone surrounding the Mo5, within 72 hrs. These observations suggest that signals are transmitted from the VN and PH to masseter motoneurons mainly through a multisynaptic circuit.
In conclusion, our findings provide the first direct evidence that neurons in the VN and PH project bilaterally to distinct populations of trigeminal motoneurons through a multisynaptic pathway. Our data are concordant with the conclusions of most physiological studies, which showed that the latencies of vestibular-elicited masseter muscle responses are too long for these responses to be mediated through direct connections between the VN and Mo5 (Tolu and Pugliatti 1993; Tolu et al. 1994, 1996; Deriu et al. 1999). Potential relays from the VN and PH to masseter motoneurons could include several regions of the pontomedullary reticular formation, particularly the reticular zone surrounding the Mo5, which has been reported to contain neurons that are components of the network responsible for the generation of rhythmic jaw movements (Donga and Lund 1991; Inoue et al 1992; Westberg et al 1998) and to be involved in modulating trigeminal motoneuron activity induced by peripheral and cortical inputs (Sessle 1977; Nozaki et al. 1986 a,b; Westberg and Olsson 1991).
The trigeminal sensory complex (TSC) might be another integrative center mediating vestibular influences on masseteric motoneurons. In addition to signals from sensory neurons, all subnuclei of the spinal trigeminal nucleus and of the principal trigeminal nucleus contain interneurons receiving a variety of convergent inputs from more than one source; cells in these regions also project directly to masseteric motoneurons (Olsson et al 1986; Westberg and Olsson 1991; Westberg et al 1995; Inoue et al. 2002). Amongst the afferents to TSC neurons, those arising from the VN and PH have been described in many previous studies (Lowick and Wolstencroft 1983; Walberg et al. 1985; Marfurt and Rajchert 1991; Buisseret-Delmas 1999; Valla et al. 2003). TSC premotor neurons have been suggested to integrate the rhythmic drive from the masticatory central pattern generator with descending cortical commands in order to optimize the temporal patterning and the relative amplitude of mandibular muscle activity (Kamogawa et al. 1994; Inoue et al. 2002). The present data raise the possibility that vestibular signals could modulate the processing of signals by TSC neurons to account for the animal’s head position and/or head movements.
Acknowledgements
Pseudorabies virus and anti-pseudorabies virus antibodies were the generous gift of Dr. Lynn Enquist of Princeton University. The authors thank Lucy Cotter, Jen-Shew Yen, and Bob Sabol for valuable technical assistance in completing this study. Funding was provided by Grants R01-DC003732 and R03-DC005911 from the National Institutes of Health (USA), as well as Ministero dell’ Università della Ricerca Scientifica e Tecnologica and Regione Autonoma della Sardegna (Italy).
References
- Aston-Jones G, Card P. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J Neurosci Meth. 2000;103:51–61. doi: 10.1016/s0165-0270(00)00295-8. [DOI] [PubMed] [Google Scholar]
- Bartha A. Experimental reduction of virulence of Aujezky’s disease. Magy Allatorov Lapja. 1961;16:42–45. [Google Scholar]
- Buisseret-Delmas C, Compoint C, Delfini C, Buisseret P. Organisation of reciprocal connections between trigeminal and vestibular nuclei in the rat. J Comp Neurol. 1999;409:153–168. doi: 10.1002/(sici)1096-9861(19990621)409:1<153::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Card JP. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev. 1998;22:685–694. doi: 10.1016/s0149-7634(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Card JP, Whealy ME, Robbins AK, Enquist LW. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- Deriu F, Podda MV, Chessa G, Tolu E. Trigeminal integration of vestibular and forelimb nerve inputs. Arch Ital Biol. 1999;137:63–73. [PubMed] [Google Scholar]
- Deriu F, Podda MV, Milia M, Chessa G, Sau G, Pastorino M, Aiello I, Tolu E. Masseter muscle activity during vestibular stimulation in man. Arch Ital Biol. 2000;138:205–215. [PubMed] [Google Scholar]
- Deriu F, Tolu E, Rothwell JC. A short latency vestibulomasseteric reflex evoked by electrical stimulation over the mastoid in healthy humans. J Physiol. 2003;553(1):267–279. doi: 10.1113/jphysiol.2003.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F, Tolu E, Rothwell JC. A sound-evoked vestibulomasseteric reflex in healthy humans. J Neurophysiol. 2005;93:2739–2751. doi: 10.1152/jn.01005.2004. [DOI] [PubMed] [Google Scholar]
- Donga R, Lund JP. Discharge patterns of trigeminal commissural last-order interneurons during fictive mastication in the rabbit. J Neurophysiol. 1991;66:1564–1578. doi: 10.1152/jn.1991.66.5.1564. [DOI] [PubMed] [Google Scholar]
- Eriksson PO. Muscle-fibre composition of the human mandibular locomotor system. Enzyme-histochemical and morphological characteristics of functionally different parts. Swed Dent J. 1982;12:1–44. [PubMed] [Google Scholar]
- Eriksson PO, Thornell LE. Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28:781–795. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus I. Masticatory muscle motor systems. Brain Res Rev. 1997;25:255–275. doi: 10.1016/s0165-0173(97)00026-x. [DOI] [PubMed] [Google Scholar]
- Godeaux E, Desmedt JE. Human masseter muscle: H-and tendon reflexes. Arch Neurol. 1975;32:229–234. doi: 10.1001/archneur.1975.00490460045005. [DOI] [PubMed] [Google Scholar]
- Herring SW, Grimm AF, Grimm BR. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979;154:563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Hickenbottom RS, Bishop B, Moriarty MT. Effects of whole body rotation on masseteric motoneuron excitability. Exp Neurol. 1985;89:442–453. doi: 10.1016/0014-4886(85)90103-7. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Card JP, Olson L. A spinal cord pathway connecting primary afferents to the segmental sympathetic outflow system. Exp Neurol. 2005;194:128–138. doi: 10.1016/j.expneurol.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Inoue T, Masuda Y, Nagashima T, Yoshikawa K, Morimoto T. Properties of a rhythmically active reticular neurons around the trigeminal motor nucleus during fictive mastication in the rat. Neurosci Res. 1992;14:275–294. doi: 10.1016/0168-0102(92)90072-k. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nozawa-Inoue K, Donga R, Yamada Y. Convergence of selected inputs from sensory afferents to trigeminal premotor neurons with possible projections to masseter motoneurons in the rabbit. Brain Res. 2002;957:183–191. doi: 10.1016/s0006-8993(02)03662-4. [DOI] [PubMed] [Google Scholar]
- Kamogawa H, Manabe K, Kondo M, Naito K. Supra- and juxtatrigeminal inhibitory premotor neurons with bifurcating axons projecting to masseter motoneurons on both sides. Brain Res. 1994;639:85–92. doi: 10.1016/0006-8993(94)91767-1. [DOI] [PubMed] [Google Scholar]
- Kevetter G, Leonard R, Newlands S, Perachio A. Central distribution of vestibular afferents that innervate the anterior or lateral semicircular canal in the mongolian gerbil. J Vestib Res. 2004;14:1–15. [PubMed] [Google Scholar]
- Kolta A, Westberg KG, Lund JP. Identification of brainstem interneurons projecting to the trigeminal motor nucleus and adjacent structures in the rabbit. J Chem Neuroanat. 2000;19:175–195. doi: 10.1016/s0891-0618(00)00061-2. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Premotor neurons for trigeminal motor nucleus neurons innervating the jaw-closing and jaw-opening muscles-differential distribution in the lower brainstem of the rat. J Comp Neurol. 1995;356:563–579. doi: 10.1002/cne.903560407. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Wolstencroft JH. Projections from brainstem nuclei to the spinal trigeminal tractus in the cat. Neuroscience. 1983;9:411–420. doi: 10.1016/0306-4522(83)90303-2. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Raichert DM. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J Comp Neurol. 1991;196:173–187. doi: 10.1002/cne.903030313. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Baker R. Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Baker R, Delgado-Garcia J. Afferent and efferent organization of the prepositus hypoglossi nucleus. Prog Brain Res. 1979;50:653–665. doi: 10.1016/S0079-6123(08)60863-8. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Konishi A, Sato M. Localization of masticatory motoneurons in the cat and rat by means of retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1975;164:105–115. doi: 10.1002/cne.901640109. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Yasui Y, Nomura S, Itoh K, Konishi A, Takada M, Kudo M. A light and electron microscopic study of premotor neurons for the trigeminal motor nucleus. J Comp Neurol. 1983;215:290–298. doi: 10.1002/cne.902150305. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Role of corticobulbar projection neurons in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986a;55:826–845. doi: 10.1152/jn.1986.55.4.826. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986b;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- Olsson KA, Landgren S, Westberg KG. Location of, and peripheral convergence on, the interneuron in the disynaptic path from the coronal gyrus of the cerebral cortex to the trigeminal motoneurons in the cat. Exp Brain Res. 1986;65:83–97. doi: 10.1007/BF00243832. [DOI] [PubMed] [Google Scholar]
- Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rats studied by anterograde transport of horseradish peroxidase conjugated to wheat germ-agglutinin. J Comp Neurol. 1988;268:91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- Rubertone JA, Mehler WR, Voogd J. The vestibular nuclear complex. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. pp. 773–796. [Google Scholar]
- Sessle BJ. Modulation of alpha and gamma trigeminal motoneurons by various peripheral stimuli. Exp Neurol. 1977;54:323–339. doi: 10.1016/0014-4886(77)90273-4. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Hirose Y, Yoshida A, Fukami H, Honma S, Bae YC. Quantitative ultrastructure of physiologically identified premotoneuron terminals in the trigeminal motor nucleus in the cat. J Comp Neurol. 2000;426:13–30. [PubMed] [Google Scholar]
- Stålberg E, Eriksson PO, Antoni L, Thornell LE. Electrophysiological study of size and fibre distribution of motor units in the human masseter and temporal muscles. Arch Oral Biol. 1986;31:521–527. doi: 10.1016/0003-9969(86)90145-7. [DOI] [PubMed] [Google Scholar]
- Strick PL, Card JP. Transneuronal mapping of neural circuits with alpha herpes viruses. In: Bolam JP, editor. Experimental Neuroanatomy: a practical approach. Oxford University Press; Oxford: 1992. pp. 81–101. [Google Scholar]
- Tolu E, Caria MA, Chessa G, Melis F, Simula ME, Podda MV, Solinas A, Deriu F. Trigeminal motoneurone responses to vestibular stimulation in the guinea pig. Arch Ital Biol. 1996;134:141–151. [PubMed] [Google Scholar]
- Tolu E, Pugliatti M. The vestibular system modulates masseter muscle activity. J Vest Res. 1993;3:163–171. [PubMed] [Google Scholar]
- Tolu E, Pugliatti M, Lacana P, Chessa G, Caria MA, Simula ME. Vestibular and somatosensory afferents modulate masseter muscle activity. J Vest Res. 1994;4:303–311. [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projection to the oral motor nuclei in the rat. J Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- Valla J, Delfini C, Diagne M, Pinganaud G, Buisseret P, Buisseret-Delmas C. Vestibulotrigeminal and vestibulospinal projections in rats: retrograde tracing coupled to glutamic acid decarboxylase immunoreactivity. Neurosci Lett. 2003;340:225–228. doi: 10.1016/s0304-3940(03)00127-7. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Sutin J. Brainstem projections to the normal and noradrenergically hyperinnervated trigeminal motor nucleus. J Comp Neurol. 1983;214:198–208. doi: 10.1002/cne.902140207. [DOI] [PubMed] [Google Scholar]
- Walberg F, Dietrics E, Nordby T. On the projections from the vestibular and peryhypoglossal nuclei to the spinal trigeminal and lateral nuclei in the cat. Brain Res. 1985;133:123–130. doi: 10.1016/0006-8993(85)90131-3. [DOI] [PubMed] [Google Scholar]
- Weijs WA. Functional somatotopic organization of motoneurons supplying the rabbit masseter muscle. J Comp Neurol. 1996;364:279–289. doi: 10.1002/(SICI)1096-9861(19960108)364:2<279::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Jüch PJW, Kwa SHS, Korfage JAM. Motor unit territories and fiber types in rabbit masseter muscle. J Dent Res. 1993;72:1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- Westberg KG, Clavelou P, Sandstrom G, Lund JP. Evidence that trigeminal brainstem interneurons form subpopulations to produce different forms of mastication in the rabbit. J Neurosci. 1998;18:6466–6479. doi: 10.1523/JNEUROSCI.18-16-06466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberg KG, Olsson KA. Integration in trigeminal premotor interneurones in the cat. Functional characteristic of neurones in the subnucleus of the oral nucleus of the spinal trigeminal tract. Exp Brain Res. 1991;84:323–339. doi: 10.1007/BF00231765. [DOI] [PubMed] [Google Scholar]
- Westberg KG, Sandstrom G, Olsson KA. Integration in trigeminal premotor interneurones in the cat. 3. Input characteristics and synaptic actions of neurons in subnucleus-gamma of the oral nucleus of the spinal trigeminal tract with a projection to the masseteric motoneurone subnucleus. Exp Brain Res. 1995;104:449–461. doi: 10.1007/BF00231979. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Klugman DK, English AW. Anatomical partitioning and nerve branching patterns in the adult rabbit masseter. Acta Anat. 1997;159:222–232. doi: 10.1159/000147988. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Carrasco DI, English AW. Differential activation of neuromuscular compartments in the rabbit masseter muscle during different oral behaviors. Exp Brain Res. 2003;150:297–307. doi: 10.1007/s00221-003-1464-y. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Lund JP. Evidence that peaks in EMG averages can sometimes be caused by inhibition of motoneurons. J Neurol. 1989;62:212–219. doi: 10.1152/jn.1989.62.1.212. [DOI] [PubMed] [Google Scholar]
- Yang M, Card JP, Tirabassi RS, Miselis RR, Enquist LW. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuitry of the rat brain is facilitated by gE mutations that reduce virulence. J Virol. 1999;73:4350–4359. doi: 10.1128/jvi.73.5.4350-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]