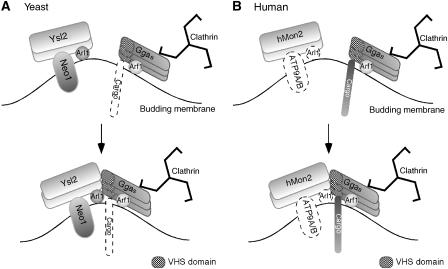
Figure 8.
Comparison of network interactions between Ysl2p/hMon2, Arl1p, Neo1p orthologues, and Gga clathrin adaptors in yeast and humans. (A) Multimeric Ysl2p, small GTPase Arl1p, and Neo1p cooperate during vesicle formation and endosome biogenesis. All direct contacts indicated in the cartoon represent experimentally supported physical interactions. Ggas can bind to clathrin and are recruited to membranes by GTP–Arf1p. Significantly, Arf1p interaction appears not to be sufficient for Gga function or localization and cargo (indicated by a dotted symbol) directly binding to Ggas through VHS has not yet been identified. Our results show that Ysl2p directly interacts with Gga1p/Gga2p through VHS; GTP–Arl1p binds to Gga2p; Neo1p is linked to Gga recruitment by virtue of a mutant allele, neo1-69, and by suppression of Δysl2; membrane-bound Gga2p interacts with itself. (B) Human homologues of the Ysl2p–Arl1p–Neo1p complex exist, but biochemical and functional links among them are unknown. Arl1 and the putative human Neo1p orthologues, ATP9A/B, are represented by dotted symbols. Cargo that directly binds to Ggas through VHS has been identified. GTP–Arf1 has a central role in the membrane docking of Ggas through GAT. Binding of Ggas to clathrin occurs as described in (A). Our new data show that hMon2 interacts with Ggas in vivo and in vitro (through VHS), and loss of hMon2 has an impact on Gga targeting.