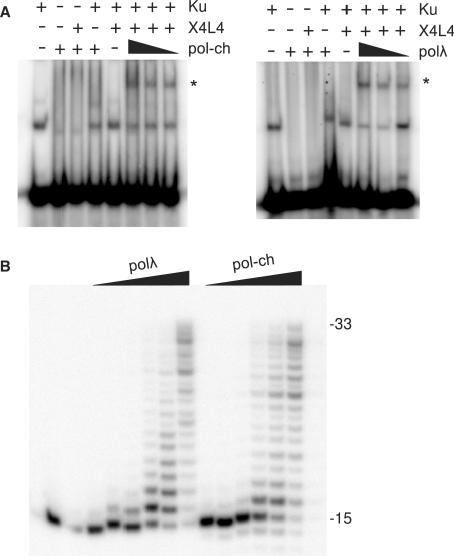
Figure 1.
Catalytic activity of a chimeric DNA polymerase and interaction with core end joining proteins. (A) A 5′-32P-end-labeled 60-base duplex (90 nM) was incubated with Ku (5 nM), X4L4 (25 nM) and 25, 50 or 100 polλ or a chimeric DNA polymerase (pol-ch) wherein the catalytic core of polλ was replaced with that of polβ (‘+’ = 100 nM). Complexes were resolved on a nondenaturing gel. The uppermost band (asterisk) is formed only when all three proteins are present, and presumably represents a stable complex of the three on DNA (11). This complex forms equally well with either polλ or pol-ch. (B) A partial duplex consisting of a 15-base primer annealed to a 33-base template (3 nM) was treated with 3.5, 7, 14, 35, 70 or 140 nM polλ or pol-ch. Following incubation for 1 h at 37°C, samples were analyzed on a sequencing gel.