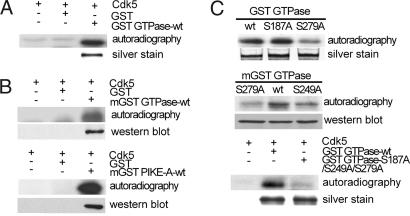
Fig. 1.
Phosphorylation of PIKE-A by Cdk5 in vitro. (A) Phosphorylation of bacteria-expressed GTPase domain of PIKE-A by Cdk5 in vitro. GST alone was used as negative control. Lower is silver staining of purified GST GTPase. (B) Phosphorylation of mammalian-expressed GST GTPase domain (mGST GTPase WT) and full-length PIKE-A (mGST PIKE-A-WT) by Cdk5 in vitro. (Upper) Kinase results. (Lower) The membrane was reprobed with anti-GST antibody. (C) Identification of Cdk5 phosphorylation sites in GTPase domain. Various GST fusion proteins purified from bacteria were assayed for phosphorylation by Cdk5 as described in A (Top and Bottom). Mammalian-expressed fusion proteins immunoprecipitated from cells were assayed for phosphorylation by Cdk5 (Middle).