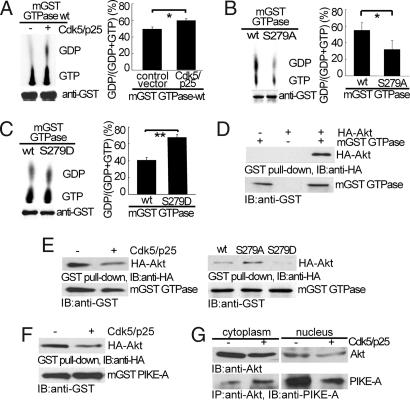
Fig. 2.
Phosphorylation by Cdk5 on PIKE-A GTPase activity and binding of PIKE-A and Akt. (A) Stimulation of PIKE-A GTPase activity by Cdk5. The hydrolysis of GTP to GDP was separated on TLC, measured by autoradiography (Upper Left) and quantified (Right). (Lower Left) Loading of mGST GTPase. (B) Reduction of GTPase activity in S279A mutant. GTPase WT and S279A mutant overexpressed in HEK293 cells were compared for their GTPase activity as described in A. (C) Increase in GTPase activity in S279D mutant. All data above were calculated as means (± SD) of three independent experiments (*, P < 0.05 and **, P < 0.01, Student's t test). (D) Interaction of PIKE GTPase domain and Akt. mGST GTPase and HA-Akt expressed in HEK293 was pulled down with glutathione sepharose 4B beads, and coprecipitated proteins were analyzed by anti-HA antibody. (E) Decreased binding of GTPase domain to Akt. The experiments were carried out as in D under the indicated conditions. (Left) Inhibition of mGST GTPase binding to Akt by Cdk5. (Right) The interaction between Akt and mGST GTPase mutants. (F) Decreased binding of Akt binding to full-length PIKE-A. Binding of Akt and full-length PIKE-A was carried out as in E. (G) Decreased binding of endogenous PIKE-A to Akt in the nucleus. After transfection, Akt was immunoprecipitated from LN-Z308 glioblastoma cells, and coprecipitated PIKE-A was detected by anti-PIKE-A antibody.