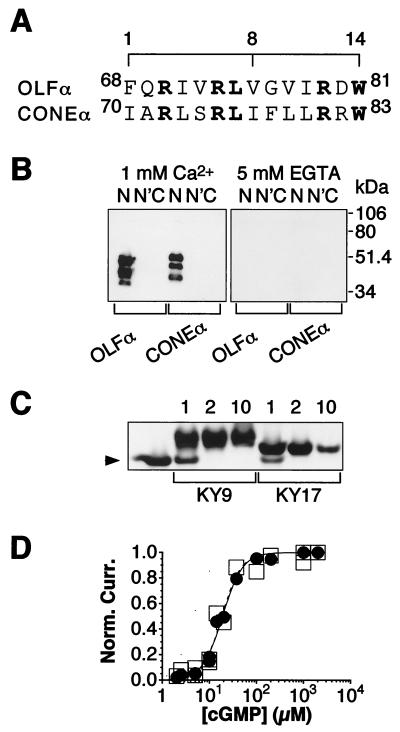
Figure 1.
Identification of a CaM-binding site on human CONEα. (A) Sequence of the CaM-binding site on human CONEα aligned with that on rat OLFα. The consensus motif of three aromatic/hydrophobic amino acids at positions 1, 8, and 14 is indicated. Boldface indicates identical residues between the two sequences. (B) Gel-overlay experiment with biotinylated CaM and GST–fusion proteins of the N and C termini of CONEα. N′, N-terminal fusion protein with the CaM-binding site deleted. As controls, the corresponding fusion proteins of OLFα were included in the experiment. After the CaM overlay, the blots were stripped and probed with an α-GST antibody, and the results indicated roughly the same amount of protein in each lane (data not shown). The calculated Mr of the OLFα and CONEα N-terminal fusion proteins are 44 and 47 kDa, respectively, and 61 and 62 kDa for the C-terminal fusion proteins. The additional bands probably represent degradation products. (C) Gel-shift experiment with a peptide (KY17) corresponding to the CaM-binding site on CONEα (residues 65–89). The peptide KY9, corresponding to the site on OLFα (residues 62–87), was included for comparison. CaM (375 pmol) and a peptide in peptide/CaM mole ratios of 1, 2, or 10 (indicated above the lanes), plus 2 mM Ca2+, was resolved on a 15% nondenaturing gel and visualized with Coomassie blue staining. The leftmost lane contains CaM but no peptide. The arrowhead indicates the position of free CaM. No shifts were observed without Ca2+ (data not shown). (D) Dose–response relation between activated current and cGMP concentration for wild-type CONEα expressed in HEK 293 cells in the presence (●) and absence (□) of 250 nM CaM, both with 50 μM Ca2+. Results from patch-clamp recordings from excised, inside-out membrane patches of the transfected cells. Membrane potential at −60 mV. Individual data points from three patches are plotted with the same symbols. Curve fits are according to the Hill equation, I/Imax = Cn/[Cn + K1/2n]. The K1/2 values in the absence or presence of CaM were 19.1 and 18.4 μM cGMP, respectively, both with n = 2.1.