Abstract
IL-2 and IL-21 are closely related cytokines that might have arisen by gene duplication. Both cytokines promote the function of effector CD8+ T cells, but their distinct effects on antigen-driven differentiation of naive CD8+ T cells into effector CD8+ T cells are not clearly understood. We found that antigen-induced expression of Eomesodermin (Eomes) and maturation of naive CD8+ T cells into granzyme B- and CD44-expressing effector CD8+ T cells was enhanced by IL-2, but, unexpectedly, suppressed by IL-21. Furthermore, IL-21 repressed expression of IL-2Ra and inhibited IL-2–mediated acquisition of a cytolytic CD8+ T-cell phenotype. Despite its inhibitory effects, IL-21 did not induce anergy, but instead potently enhanced the capacity of cells to mediate tumor regression upon adoptive transfer. In contrast, IL-2 impaired the subsequent antitumor function of transferred cells. Gene expression studies revealed a distinct IL-21 program that was characterized phenotypically by increased expression of L-selectin and functionally by enhanced antitumor immunity that was not reversed by secondary in vitro stimulation with antigen and IL-2. Thus, the efficacy of CD8+ T cells for adoptive immunotherapy can be influenced by opposing differentiation programs conferred by IL-2 and IL-21, a finding with important implications for the development of cellular cancer therapies.
Introduction
Cytokine priming signals direct CD8+ T cells to acquire specific qualities that can influence their ability to mediate effective immune responses following adoptive transfer.1–3 Understanding the effects of these signals on CD8+ T-cell function is important to the development of effective adoptive immunotherapy. CD8+ T cells activated with IL-2 can lyse tumor targets, which has led to use of IL-2 for the generation of T cells for cell transfer therapies for cancer.4 Although IL-2 was identified in 19765 and approved for clinical use in 1992, our appreciation of the full range of its actions is still evolving. IL-2 potently promotes activation and proliferation of CD8+ T cells,6,7 and it can induce cancer regression when administered to patients.4 However, IL-2 also induces activation-induced cell death (AICD) and the development of suppressive T regulatory (Treg) cells.6,8
IL-21 is the most recently identified member of the family of cytokines that share the common cytokine receptor γ-chain with IL-2.9,10 IL2 and IL21 are adjacent genes, separated by approximately 180 kb in humans and 95 kb in mice, and they have similar intron and exon structures, suggesting that they arose by gene duplication.9,11 IL-21R is most closely related to the IL-2Rβ, and IL-2 and IL-21 have significant structural homology.9,12 Like IL-2, IL-21 can promote the function of effector CD8+ T cells,10,13,14 but the effect of IL-21 on the differentiation of naive CD8+ T cells into effector CD8+ T cells is not clear.
We found that IL-2 and IL-21 mediated opposing effects on antigen-induced CD8+ T-cell differentiation. Eomesodermin (Eomes) expression and development of cytolytic function was promoted by IL-2, but suppressed by IL-21. However, IL-21 did not induce anergy, but instead conferred a distinct gene expression program characterized by increased expression of L-selectin and enhanced antitumor activity following adoptive transfer. In contrast, priming with IL-2 actually impaired the subsequent function of tumor-specific T cells for adoptive immunotherapy, an effect that was significantly reversed by addition of IL-21. These findings demonstrate an antagonistic relationship between the actions of IL-2 and IL-21 on the development of effector CD8+ T cells and have important implications for the generation of T cells for adoptive immunotherapy.
Methods
Mice and tumor lines
Pmel-1 TCR-transgenic mice15 were crossed with C57BL/6 Thy1.1 congenic mice (The Jackson Laboratories, Bar Harbor, ME) to yield mice expressing both the pmel-1 TCR and Thy1.1 congenic marker. Il21r−/− mice16 were backcrossed 7 generations with C57BL/6 mice. Wild-type C57BL/6 mice were from The Jackson Laboratories. All animal experiments were approved by the NCI Animal Ethics Committee. B16 melanoma and MCA-205 tumor cell lines (NCI Tumor Repository) were maintained in culture media.15
CD8+ T-cell priming and restimulation
CD8+ T-cell splenocytes were isolated using positive or negative magnetic bead selection (Miltenyi Biotec, Auburn, CA). In indicated experiments, naive cells were isolated by additional depletion of CD44high cells using biotinylated anti-CD44 antibodies (BD Biosciences, Franklin Lakes, NJ) and antibiotin magnetic beads (Miltenyi Biotec). Pmel-1 CD8+ T cells were primed with irradiated splenocytes (3000 cGy) pulsed with 1 μM hgp10025-33.15 Secondary responses were initiated in the same manner, with the exception of the microarray analysis, in which antigen-primed pmel-1 CD8+ T cells were restimulated for 4 hours with plate-bound anti-CD3 and anti-CD28 to avoid contaminating feeder cells. Wild-type CD8+ T cells were stimulated using 2 μg/mL plate-bound anti-CD3 and 1 μg/mL soluble anti-CD28. Recombinant human IL-2 (Novartis, Emeryville, CA), recombinant human IL-15 (Peprotech, Rocky Hill, NJ), or recombinant murine IL-21 (R&D Systems, Minneapolis, MN) were added to the culture conditions as indicated.
Real-time reverse transcription–polymerase chain reaction
RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA). cDNA was generated by reverse transcription (RT; Invitrogen). Real-time polymerase chain reaction (PCR) was performed using commercially available probes and primers for the indicated genes (Applied Biosystems, Foster City, CA) and a Prism 7900HT (Applied Biosystems). The levels of gene expression were calculated relative to the housekeeping genes β-actin or Rpl7.
Flow cytometry
Cells were labeled with fluorochrome-conjugated antibodies specific for the target indicated. Antibodies for CD44, L-selectin, and Thy1.1 were from BD Biosciences. Intracellular staining was performed by fixing and permeabilizing cells (BD Biosciences) and labeling with antibodies for Granzyme B (eBioscience, San Diego, CA). Samples were collected using a FACSCalibur flow cytometer and analyzed using FlowJo software (TreeStar, Ashland, OR).
Expansion, proliferation, cytokine release, and cytolytic assays
In vitro expansion was assessed by enumeration of viable cells using Trypan Blue exclusion. Proliferation assays were conducted by labeling pmel-1 Thy1.1 CD8+ T cells with CFSE (Invitrogen) and stimulating with 1 μM hgp10025-33-pulsed, 3000 cGy-irradiated splenocytes. Cells were harvested on the days indicated and labeled with fluorochrome-conjugated antibodies, and fixed. Flow cytometry was then performed on samples at the completion of the experiments. Cytokine release assays were performed with pmel-1 CD8+ T cells after antigen priming and 4 days of culture with 10 ng/mL of the indicated cytokine. Primed cells were then washed and cocultured overnight with irradiated (3000 cGy) splenocytes pulsed with 1 μM hgp10025-33. IFN-γ and IL-2 concentrations in the coculture supernatants were determined by enzyme-linked immunosorbent assay (ELISA; Pierce Endogen, Rockford, IL). Chromium release cytolysis assays were performed as described17 6 days after antigen priming of pmel-1 CD8+ T cells with 10 ng/mL of the indicated cytokine.
Microarray analysis
Pmel-1 CD8+ T cells, antigen-primed in 10 ng/mL of the indicated cytokine or in CM without cytokine added (control) for 4 days, were washed and restimulated in cytokine neutral conditions for 4 hours. RNA was isolated using Trizol Reagent (Invitrogen). Additional purification was performed using RNeasy columns (Qiagen, Valencia, CA). RNA was indirectly labeled via a single round of linear amplification with Amino Allyl MessageAmp II reagents (Ambion, Austin, TX) with the control cells serving as a reference. The labeled experimental RNA was combined with labeled control RNA and hybridized overnight to 38 000-spot long oligonucleotide mouse exonic evidence-based oligonucleotide (MEEBO) arrays (NCI Microarray Core Facility). Arrays were scanned using a GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA) and data were acquired with Genepix Pro 5.1 (Molecular Devices). The data files were imported into GeneSpring GX 7.3.1 (Silicon Genetics, Redwood City, CA) for all analyses. A Welch 1-way analysis of variance (ANOVA) by cytokine was performed using the geometric average of 3 arrays for each cytokine to determine spots with P less than .05. The resulting data from the ANOVA were then used for a Pearson correlation hierarchical clustering between conditions and genes. These data sets are available at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) as accession no. GSE10403.
Adoptive cell transfer
Mice 6 to 8 weeks of age were injected subcutaneously with 5 × 105 B16 tumor cells. At 9 days after tumor injection, they were irradiated with 500 cGy and treated with adoptive transfer of pmel-1 CD8+ T cells or pmel-1 Thy1.1 CD8+ T cells. Recombinant fowlpox or vaccinia virus expressing human gp10015 2 × 107 plaque forming units (PFUs) was concomitantly administered intravenously, and IL-2 was given as 600 000 IU intraperitoneally twice daily for a total of 6 doses. IL-2 was administered to all mice that received cell transfer, regardless of the cytokine present during antigen priming. Treatment groups had 5 to 10 mice each. Blinded, serial tumor measurements were obtained and the products of the perpendicular diameters were plotted. The number of transferred cells present in recipient mouse spleens was determined by enumeration of splenocytes and use of flow cytometry to assess the frequency of Thy1.1 splenocytes. The frequency of Thy1.1 cells was multiplied by the number of splenocytes to derive the absolute number of Thy1.1 splenocytes. Spleens from 3 mice per group were assessed individually.
Statistics
Statistical analyses comparing values between conditions were performed using one-way ANOVA with the Bonferroni multiple comparison test. Tumor growth curves were analyzed using the Wilcoxon matched pairs test. These analyses were performed using Prism for Macintosh v4.0a software.
Results
Antigen-induced differentiation of naive CD8+ T cells into effector CD8+ T cells is enhanced by IL-2 and IL-15, but suppressed by IL-21
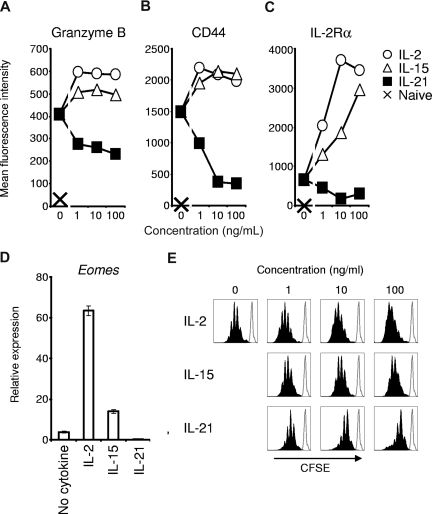
We first investigated the effects of IL-2 and IL-21 on antigen-driven acquisition of an effector CD8+ T-cell phenotype. Comparison was made to cells primed without additional cytokine or with IL-15, a cytokine that shares IL-2Rβ and γc with IL-2, but does not promote activation-induced cell death or generation of Treg cells.6,8 Pmel-1 TCR-transgenic CD8+ T cells were studied to permit subsequent assessment of antitumor immunity against established, unmanipulated B16 melanoma expressing the self/tumor antigen gp100.15 We first assessed expression of the cytolytic molecule, granzyme B, and the adhesion molecule, CD44, after antigen priming with IL-2, IL-15, or IL-21. Granzyme B mediates caspase-dependent and caspase-independent target cell killing and is increasingly expressed as CD8+ T cells mature into full effector cells.18–20 CD44 is expressed at low levels in the earliest stages of postthymic CD8+ T-cell development (ie, naive cell and memory stem cell), but its expression rapidly and progressively increases with maturation.1,20,21 Granzyme B induction by antigen priming was enhanced by IL-2 and IL-15 but was reduced by IL-21 (Figure 1A). In contrast to IL-2 and IL-15, IL-21 inhibited CD44 expression (Figure 1B), and high concentrations of IL-21 induced a CD44low/intermediate phenotype, similar to the early differentiation phenotype of memory stem cells.21 This phenotype was the result of the direct action of IL-21 on CD8+ T cells, as IL-21 also inhibited expression of granzyme B and CD44 when CD8+ T cells were primed with peptide-pulsed Il21r−/− splenocytes (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Figure 1.
Antigen-induced acquisition of effector CD8+ T-cell phenotype is promoted by IL-2 and IL-15 but suppressed by IL-21. Naive pmel-1 Thy1.1 CD8+ T cells were primed with cognate antigen and the indicated cytokine for 3 days. (A-C) Flow cytometric determination of granzyme B, CD44, and IL-2Rα expression by Thy1.1 lymphocytes. (D) Real-time RT-PCR determination of Eomes expression 3 days after priming in 10 ng/mL of the indicated cytokine. Error bars indicate the standard error of the mean. (E) CFSE dilution 4 days after antigen priming of naive pmel-1 Thy1.1 CD8+ T cells with the indicated cytokine. Histograms are gated on Thy1.1 cells. The open histogram overlay indicates CFSE labeling prior to stimulation.
Given the contrasting actions of IL-2 and IL-21 on expression of granzyme B and CD44, we investigated the effect of IL-21 on antigen-induced expression of IL-2Rα, which is required for formation of the high-affinity IL-2 receptor. Unlike IL-2 and IL-15, IL-21 inhibited antigen-induced IL-2Rα expression (Figure 1C). This finding indicated that, in distinction to IL-2, which acts through a positive feedback loop to promote IL-2Rα expression and perpetuate effector CD8+ T-cell development,7 IL-21 repressed IL-2Rα expression, potentially counteracting the effects of IL-2.
We assessed the effects of IL-2 and IL-21 on transcriptional regulation of effector CD8+ T-cell differentiation by determining levels of Eomes mRNA following antigen priming. Eomes is a T-box transcription factor that confers cytolytic lymphocyte lineage characteristics.22,23 Eomes expression was augmented by IL-2 and IL-15 but suppressed by IL-21 (Figure 1D), an effect observed across a broad range of concentrations (Figure S2). Taken together, these data indicated a distinct negative regulatory influence of IL-21 on antigen-driven effector differentiation. These inhibitory actions were not related to impaired cell viability (Figure S3). However, IL-21 did suppress antigen-driven clonal proliferation and did not support sustained CD8+ T-cell expansion (Figure 1E).
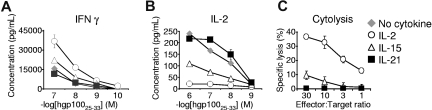
To test the functional significance of priming with these cytokines, cytokine release and cytotoxicity assays were performed. As naive CD8+ T cells mature into effector CD8+ T cells, their capacity to release IFN-γ increases, whereas their ability to secrete IL-2 decreases.20,24,25 Consistent with a less differentiated phenotype, cells antigen-primed with IL-21 secreted less IFN-γ (Figure 2A) and more IL-2 (Figure 2B) than their IL-2– or IL-15–primed counterparts. Furthermore, cells that were primed in the presence of IL-2 or IL-15 mediated significant antigen-specific target killing, but cells that received primary stimulation with IL-21 showed minimal, if any, specific cytolytic activity (Figure 2C). Taken together with the phenotypic observations, these data demonstrate that antigen-induced differentiation of CD8+ T cells into effector CD8+ T cells was augmented by IL-2 and IL-15, but suppressed by IL-21.
Figure 2.
In contrast to IL-2 and IL-15, IL-21 does not promote acquisition of effector CD8+ T-cell function. (A,B) Pmel-1 CD8+ T cells were antigen-primed with 10 ng/mL of the indicated cytokine for 4 days, washed, and cocultured overnight with irradiated splenocytes pulsed with hgp10025-33. IFN-γ and IL-2 concentrations in the supernatants were determined by ELISA. (C) Chromium release cytolysis assay showing specific target killing by pmel-1 CD8+ T cells antigen-primed with 10 ng/mL of the indicated cytokine.
The function of CD8+ T cells for adoptive immunotherapy is augmented by priming with IL-21 and impaired by priming with IL-2 or IL-15
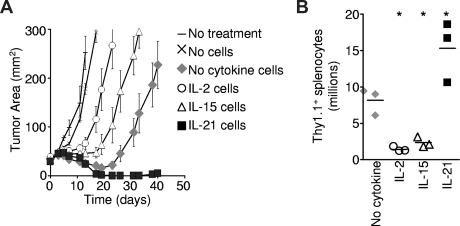
To determine the impact of priming with IL-2, IL-15, or IL-21 on the ensuing antitumor function of T cells for adoptive transfer, pmel-1 CD8+ T cells were antigen-primed in the presence of the indicated cytokines and adoptively transferred into B16 melanoma-bearing hosts. The transferred cells were restimulated in vivo with specific vaccination and exogenously administered IL-2.15 Cells primed with IL-15 mediated greater tumor destruction than cells primed with IL-2 (P = .012; Figure 3A), and these results are consistent with prior studies.17,26 However, both IL-2– and IL-15–primed cells demonstrated less tumor destruction than control cells primed without any cytokine added (P = .039 and P = .001, respectively). In contrast, IL-21–primed cells displayed significantly more potent antitumor responses than control cells (P = .020), causing sustained tumor regression that continued until detectable tumor was eliminated from all treated mice (Figure 3A). These responses were durable with only 1 of 7 mice developing tumor recurrence in 44 days of observation. This enhanced antitumor response was associated with greater numbers of transferred cells in recipient mouse spleens 12 days after transfer (Figure 3B). Thus, IL-21, present only during priming in vitro, programmed cells for a more robust immune response and greater antitumor activity in vivo. Like IL-2–primed cells,15 IL-21–primed cells required administration of exogenous IL-2 for optimal antitumor function (C.S.H., unpublished data, 2008). Regardless of the priming cytokine, transferred cells differentiated into granzyme B+ effector cells in vivo (Figure S4A). In contrast to IL-21–primed cells, cells programmed with IL-2 or IL-15 displayed decreased cell numbers (Figure 3B) and impaired tumor destruction following adoptive transfer (Figure 3A).
Figure 3.
The antitumor efficacy of CD8+ T cells for adoptive transfer is impaired by IL-2 and IL-15, but enhanced by IL-21. Pmel-1 CD8+ T cells were primed with cognate antigen and 10 ng/mL of the specified cytokine for 4 days then adoptively transferred into tumor-bearing hosts. Vaccination and exogenous IL-2 were administered to mice in all but the “No treatment” group. (A) Tumor response to adoptive transfer of 2.5 × 105 cells. The cytokine present during priming is indicated. Error bars reflect the standard error of the mean. (B) Pmel-1 Thy1.1 CD8+ T cells were antigen-primed with 10 ng/mL of the indicated cytokine. At 4 days after priming, 5 × 105 cells per mouse were infused, and vaccine and IL-2 were administered. The number of Thy1.1 splenocytes was determined 12 days after transfer. The scatter plots indicate individual mice. The horizontal lines represent the means. *P < .05 compared with “No cytokine.”
IL-21 suppresses IL-2–induced effector CD8+ T-cell differentiation
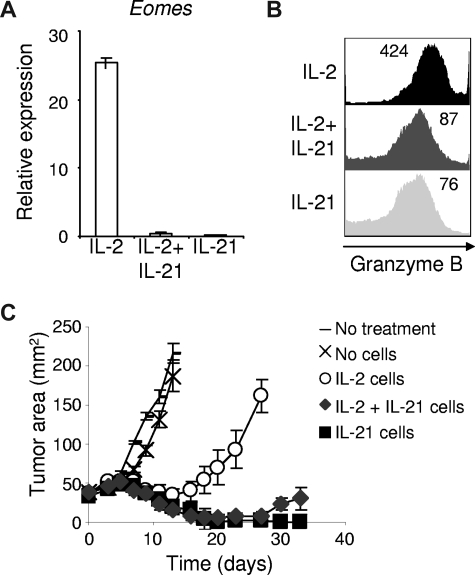
IL-2 and IL-21 had opposing effects on antigen-driven differentiation of CD8+ T cells and on the subsequent ability of these cells to induce tumor regression. To determine if IL-2 and IL-21 have antagonistic actions on effector CD8+ T-cell differentiation, we combined the 2 cytokines during antigen priming and assessed expression of Eomes and granzyme B. Induction of these molecules by IL-2 was potently suppressed by IL-21 (Figure 4A,B) and correlated with enhanced antitumor efficacy upon adoptive transfer (P = .001; Figure 4C). Taken together, these findings indicate that IL-21 prevented IL-2–induced acquisition of an effector CD8+ T-cell phenotype, and that IL-21 significantly “rescued” CD8+ T cells for adoptive immunotherapy from the negative impact of priming with IL-2.
Figure 4.
IL-21 suppresses IL-2–induced effector CD8+ T-cell differentiation and substantially prevents IL-2–mediated impairment of CD8+ T cells for adoptive transfer. Naive pmel-1 CD8+ T cells were antigen-primed with 10 ng/mL IL-2, IL-21, or 10 ng/mL IL-2 combined with 10 ng/mL IL-21. (A) Eomes expression was determined by RT-PCR 3 days following priming. (B) Granzyme B expression was assessed by flow cytometry 3 days after priming. The mean fluorescence intensity is indicated. (C) Pmel-1 CD8+ T cells were primed with cognate antigen and 10 ng/mL of the indicated cytokine(s). After 4 days, 5 × 105 cells per mouse were adoptively transferred into tumor-bearing recipients. Vaccine and IL-2 were administered to all but the “No treatment” group. Tumor responses were assessed with serial measurements. Error bars represent the standard error of the mean.
IL-21 confers a distinct gene expression program to CD8+ T cells
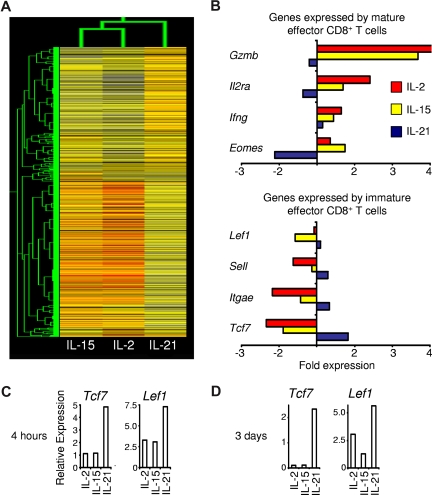
Given the contrasting actions of IL-21 with those of IL-2 and IL-15, we investigated if the cytokines confer distinct gene expression programs. After antigen priming, cells that were generated in each cytokine were restimulated in cytokine-neutral conditions and gene expression was assessed by cDNA microarray. Hierarchical cluster analysis demonstrated greater relatedness between the gene expression patterns of cells that were primed with IL-2 and IL-15 than between either IL-2 or IL-15 and IL-21 (Figure 5A). These results were consistent with observations from profiling of gene induction by IL-2 and IL-15, which both activate Stat5a and Stat5b as their major signal transducer and activator of transcription (STAT) proteins,27,28 and showed that IL-21, which signals primarily by activating Stat1 and Stat3,29 imparted a distinct program. Expression of Gzmb, Il2ra, Ifng, and Eomes, genes expressed by mature effector CD8+ T cells, was higher in cells primed with IL-2 or IL-15 than in cells primed with IL-21. Conversely, expression of Lef1, Sell, Itgae, and Tcf7, genes linked to immature effector CD8+ T cells, was greater in IL-21–primed cells than in IL-2– and IL-15–primed cells (Figure 5B). Real-time RT-PCR was used to validate observed differences in the expression of the genes encoding T-cell factor 1 (Tcf7) and Lymphoid enhancer binding factor 1 (Lef1; Figure 5C). These transcription factors were selected for validation because of their differential expression on microarray, their importance regulating hematopoietic stem cell self-renewal and T-cell development, and their diminished expression in mature effector T cells.30–33 At 3 days after restimulation with anti-CD3/anti-CD28 but without cytokine, these differences persisted (Figure 5D), suggesting maintenance of a distinct IL-21 gene expression program.
Figure 5.
Antigen priming with IL-21 imparts CD8+ T cells with a distinct gene expression program. Pmel-1 CD8+ T cells were antigen-primed with the cytokine indicated or without cytokine for 4 days, then restimulated for 4 hours without cytokine. Microarray gene expression analysis was then performed. Expression levels relative to the reference cells primed without cytokine are indicated. (A) Dendrogram showing the relatedness of gene expression patterns. (B) Microarray expression of selected genes associated with mature and immature effector CD8+ T cells. (C,D) Wild-type CD8+ T cells were stimulated with anti-CD3/anti-CD28 and 10 ng/mL of the indicated cytokine for 3 days and then restimulated in cytokine-neutral conditions for (C) 4 hours or (D) 3 days before real-time RT-PCR analysis. Expression of Tcf7 and Lef1 relative to β-actin is shown.
IL-21 programs CD8+ T cells to express L-selectin
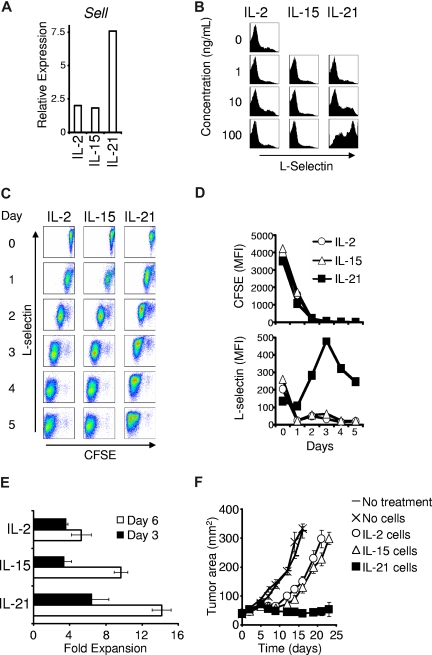
To characterize the impact of this gene expression program on subsequent CD8+ T-cell differentiation, we assessed expression of L-selectin (encoded by the Sell gene), during the secondary response to antigen. L-selectin mediates lymph node trafficking and distinguishes central memory T cells (L-selectin+ CD44high) from effector memory and effector cells (L-selectin− CD44high).34,35 The in vivo efficacy of adoptively transferred CD8+ T cells is impaired by L-selectin deficiency and augmented by enhanced expression.17,20,26,35 In response to secondary stimulation, cells primed in the presence of IL-21 exhibited greater Sell expression than cells primed in the presence of IL-2 or IL-15 (Figure 6A). Similarly, 3 days after restimulation with antigen and IL-2, cells antigen-primed with 10 ng/mL or 100 ng/mL IL-21 displayed greater L-selectin than cells antigen-primed with no cytokine, IL-2, or IL-15 (Figure 6B).
Figure 6.
Antigen priming with IL-21 directs CD8+ T cells to express L-selectin during secondary stimulation. (A) Wild-type CD8+ T cells were anti-CD3/anti-CD28 stimulated with 10 ng/mL of the indicated cytokine for 3 days. Sell expression relative to β-actin expression was determined 4 hours after restimulation in cytokine-neutral conditions. (B) Four days after antigen priming with the indicated cytokine, pmel-1 Thy1.1 CD8+ T cells were restimulated with cognate antigen and 10 ng/mL IL-2 (regardless of the initial priming cytokine). Flow cytometry was performed 3 days after initiating the secondary response and histograms of L-selectin expression are displayed. The indicated cytokines and concentrations refer to the initial priming conditions. (C) Dot plots showing CFSE dilution and L-selectin expression by pmel-1 Thy1.1 CD8+ T cells that were antigen-primed with 100 ng/mL of the indicated cytokine for 4 days, labeled with CFSE, and restimulated with antigen and 10 ng/mL IL-2 (regardless of the cytokine present during initial priming). (D) Graphs of the mean fluorescence intensity of CFSE and L-selectin during restimulation. (E) Secondary expansion of cells antigen-primed with 10 ng/mL of the indicated cytokine for 4 days then restimulated with antigen and 10 ng/mL IL-2. Error bars indicate the standard error of the mean. (F) Tumor regression in response to adoptive transfer of 106 pmel-1 CD8+ T cells primed in 10 ng/mL of the indicated cytokine for 4 days, restimulated with antigen and IL-2 for 6 days, and then adoptively transferred. Vaccine and IL-2 were administered to mice in all except the “No treatment” group. Error bars reflect the standard error of the mean.
We further investigated this finding by assessing the effect of secondary proliferation on L-selectin expression. After priming with IL-2, IL-15, or IL-21, cells were CFSE-labeled and restimulated with antigen and IL-2. CFSE dilution and L-selectin expression were simultaneously assessed on sequential days. CFSE diluted at a similar rate regardless of the cytokine that was present during priming (Figure 6C,D). L-selectin expression was similar in all groups prior to restimulation but decreased precipitously with cell division in cells primed with IL-2 or IL-15. In contrast, cells primed with IL-21 exhibited increased L-selectin expression (Figure 6C,D). Cells in all groups, regardless of the priming cytokine, acquired expression of granzyme B after restimulation (Figure S4B). IL-21–primed cells underwent greater secondary expansion than IL-2– or IL-15–primed cells (Figure 6E). Thus, antigen priming with IL-21 generated cells with greater potential for L-selectin expression and secondary expansion.
We tested if the functional program conferred by initial priming with IL-2, IL-15, or IL-21 was maintained even after in vitro restimulation with antigen and IL-2. Cells that had been antigen-primed with IL-2, IL-15, or IL-21 were expanded in vitro with cognate antigen and IL-2 for 6 days, then adoptively transferred into tumor-bearing mice. Cells initially primed with IL-21 maintained superior capacity to induce tumor regression (P = .027 compared with IL-2, P = .014 compared with IL-15; Figure 6F). This result suggested that priming with IL-21 conferred a program for superior antitumor immunity that was not reversible by secondary in vitro stimulation with antigen and IL-2.
Discussion
Adoptive transfer of tumor-specific T cells is a promising cancer treatment.3,36–42 In a recent clinical trial, infusion of tumor-reactive T cells induced regression of metastatic melanoma in approximately 50% of patients.39 Tumor responses to adoptive immunotherapy are a function not only of T-cell specificity, but also of the ability of cells to proliferate and survive following transfer.20,26,43–48 Herein we report that the magnitude and antitumor efficacy of CD8+ T-cell responses following adoptive transfer were potently enhanced by priming cells with IL-21 but, unexpectedly, impaired by priming cells with IL-2.
IL-2 promotes T-cell proliferation,5 induces tumor-killing lymphocytes,4 and causes regression of melanoma and kidney cancer in patients.49 Thus, for more than 20 years, it has been the primary cytokine for generating lymphocytes for adoptive immunotherapy. However, the actions of IL-2 on T cells are more complex than initially appreciated, as it can induce AICD and is required for development of Treg cells.6,8 In this study, we found that IL-2 augmented antigen-induced CD8+ T-cell expression of Eomes and acquisition of effector CD8+ T-cell phenotype and function.20 Although IL-2 enhanced cytolytic function, it had a negative impact on the ability of cells to induce tumor regression following adoptive transfer. This counterintuitive finding is consistent with work demonstrating an inverse relationship between CD8+ T-cell maturation into cytolytic cells before infusion and tumor regression after cell transfer.20 Similar findings are emerging from studies of tumor-reactive T cells administered to patients with cancer. CD27 expression and telomere length, indicators of an early state of effector T-cell differentiation,3,50 correlate positively with subsequent persistence of transferred cells in peripheral blood and with complete tumor regression.43–45,47 Thus, mounting evidence indicates that the differentiation of CD8+ T cells into cytolytic effector cells has a negative impact on their ability to induce tumor regression upon adoptive transfer, and a cytokine that can suppress this process might enhance the efficacy of cells for adoptive immunotherapy.
We report that IL-21 had the opposite effect from IL-2 on antigen-induced CD8+ T-cell differentiation. IL-21 repressed Eomes expression and suppressed differentiation of naive CD8+ T cells into cytolytic CD8+ T cells. This inhibition of effector CD8+ T-cell development did not prevent effector differentiation following adoptive transfer, and it was associated with enhanced ability of transferred cells to undergo secondary expansion and to mediate tumor regression. Furthermore, IL-21 antagonized IL-2–mediated induction of effector CD8+ T cells and counteracted the negative impact of IL-2 on ensuing antitumor efficacy. These findings were unexpected given that IL-21 can cooperate with IL-7 or IL-15 to enhance proliferation and function of naive CD8+ T cells, and that IL-21 can promote the antitumor function of effector CD8+ T cells.14 However, they are consistent with reports that IL-21 can (1) preserve the early differentiation CD28+ phenotype of antigen- and IL-15–stimulated human CD8+ T cells,51,52 (2) suppress Th1 effector development via repression of Eomes,53,54 and (3) inhibit dendritic cell maturation and activation thus impairing T-cell activation.55,56 IL-21 has also been reported to promote granzyme B expression57 and terminal differentiation16,58,59 in B cells, and to enhance proliferation of anti-CD3–stimulated T-cell cultures9 and cell killing by T cells in mixed lymphocyte reactions,60 suggesting that its effects are context dependent, varying in B and T cells and with differentiation state and costimulatory signals. One recent report indicates that IL-21 can enhance antigen-induced CD8+ T-cell acquisition of cytolytic function,61 but interpretation of this finding is confounded by use of a system in which IL-2 did not promote acquisition of cytolytic function by CD8+ T cells, a well-established effect of IL-2 in wide-ranging conditions and models.4,6,7,62
We found that IL-21 suppressed antigen-induced acquisition of cytolytic CD8+ T-cell characteristics at the levels of transcriptional regulation, phenotype expression, and cytolytic function, and that this inhibition resulted in superior antitumor immunity following adoptive transfer. This enhanced antitumor efficacy was associated with a distinct gene expression program distinguished by elevated expression of L-selectin, an adhesion molecule that mediates extracellular extravasation in the high endothelial venules of lymph nodes and that is necessary for optimal efficacy of adoptively transferred tumor-specific CD8+ T cells.20,21,26,35,63,64 L-selectin expression and antitumor immunity are promoted by culture in IL-15 as compared with IL-2.17,26,65 However, we found that both IL-2 and IL-15 programmed cells to rapidly decrease L-selectin expression upon restimulation. In contrast, cells primed with IL-21 expressed increased L-selectin upon secondary antigen encounter, likely contributing to their greater posttransfer expansion and tumor destruction.
In this study, we identified an unexpected dichotomy in the actions of IL-2 and IL-21 on antigen-induced CD8+ T-cell differentiation and subsequent function in adoptive immunotherapy. Although IL-2 promoted CD8+ T-cell proliferation and acquisition of cytolytic characteristics, it impaired the antitumor activity of CD8+ T cells for adoptive immunotherapy. In contrast, IL-21, which suppressed antigen-induced CD8+ T-cell acquisition of cytolytic effector lineage characteristics, increased the antitumor efficacy of cells for adoptive transfer. These findings have important implications for cancer therapy with T cells derived from peripheral blood and genetically engineered to express a tumor-specific TCR.40 They indicate that IL-2, a cytokine commonly used for T-cell transduction,37,40 has a detrimental effect on T-cell efficacy and that this action might be countered by IL-21. Efforts to use cytokines to direct development of more effective CD8+ T cells for adoptive immunotherapy are ongoing.
Supplementary Material
Acknowledgments
We thank Zhiya Yu, Dave Jones, and Shirin Treadwell for their assistance.
This research was supported by the Intramural Research Program, NCI, and NHLBI at NIH.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.S.H. designed and performed research, and wrote the paper; R.S., C.M.P., L.G., K.K., D.C., and C.K. designed and performed research; W.J.L. wrote the paper; S.A.R. and N.P.R. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian S. Hinrichs or Nicholas P. Restifo, NCI, NIH, Clinical Research Center, Rm 3-5816, Bethesda, MD, 20892-1502; e-mail: christian_hinrichs@nih.gov or restifo@nih.gov.
References
- 1.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 7.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 10.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. doi: 10.1146/annurev.immunol.26.021607.090316. Prepublished on November 8, 2007, as DOI 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 11.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 12.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 14.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 19.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 22.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 23.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 24.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovanen PE, Rosenwald A, Fu J, et al. Analysis of gamma c-family cytokine target genes: identification of dual-specificity phosphatase 5 (DUSP5) as a regulator of mitogen-activated protein kinase activity in interleukin-2 signaling. J Biol Chem. 2003;278:5205–5213. doi: 10.1074/jbc.M209015200. [DOI] [PubMed] [Google Scholar]
- 28.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 29.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 31.Verbeek S, Izon D, Hofhuis F, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 32.Duncan AW, Rattis FM, DiMascio LN, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 33.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 34.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 37.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JC, Childs R. Immunotherapy for renal cell cancer. J Clin Oncol. 2006;24:5576–5583. doi: 10.1200/JCO.2006.08.3774. [DOI] [PubMed] [Google Scholar]
- 39.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. doi: 10.1016/j.ymthe.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Shen X, Zhou J, Hathcock KS, et al. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother (1997) 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Kerstann KW, Ahmadzadeh M, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother (1997) 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Khong HT, Dudley ME, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother (1997) 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 50.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–212. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 52.Alves NL, Arosa FA, van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol. 2005;175:755–762. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 53.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 54.Wurster AL, Rodgers VL, Satoskar AR, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 56.Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Ruckert R. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol. 2003;121:1379–1382. doi: 10.1046/j.1523-1747.2003.12603.x. [DOI] [PubMed] [Google Scholar]
- 57.Jahrsdorfer B, Blackwell SE, Wooldridge JE, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712–2719. doi: 10.1182/blood-2006-03-014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 59.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 60.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 61.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins MK. Peripheral T-lymphocyte responses and function. In: Paul WE, editor. Fundamental Immunology. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 303–319. [Google Scholar]
- 63.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 64.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 65.Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.