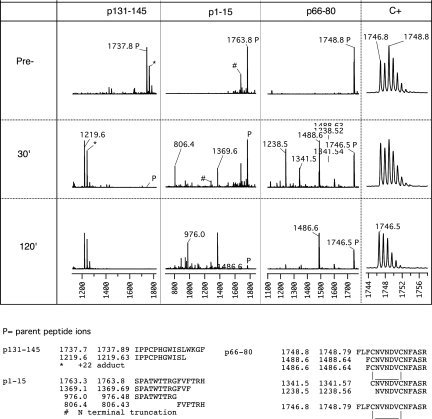
Figure 3.
MALDI-TOF analysis of fragments generated in the course of Cathepsin D digestion of three α3(IV)NC1 peptides. The first three panels of the top section show MALDI-TOF mass spectrograms of Cathepsin D digests of three α3(IV)NC1 peptides selected to illustrate the patterns of digestion observed. For each peptide, spectrograms were taken before (top) and after 30 or 120 min incubation with protease at 37°C. Peptide p131-145 (m/z 1737.89) is highly scissile because it is completely cleaved at 30 min with disappearance of the parent ion and appearance of a single major fragment (the ions marked as +22 adducts are likely to be peptides that retain a protecting group because they were observed even after extensive desalting). The sequence of the fragment deduced from its m/z is shown in the bottom row and allows inference of SL|WK as the highly scissile peptide bond. Peptide p1-15 is just scissile because it is <50% digested at 30 min with two major fragments indicative of two scissile bonds, and some parent remains even at 120 min. Digestion of p66-80 appears only slightly more complete at 30 min than p1-15, but careful inspection of the isotope pattern (far right) of the parent ion showed the initial parent to comprise two populations with m/z 2 mass units apart with subsequent depletion of only the heavier form. Comparison of freshly oxidized and reduced peptide and acetylated p66-80 confirmed that the reduced form was highly scissile and the oxidized form (with intramolecular disulfide loop) was highly resistant (data not shown).