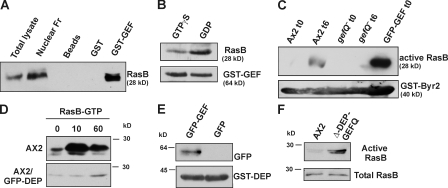
Figure 5.
RasB activation by RasGEF Q and during development. (A) Physical association of GST-GEF and RasB. Glutathione Sepharose beads coated with either GST-GEF or GST or uncoated with any protein were incubated with AX2 cell lysates and pulldown eluates were immunoblotted with RasB antibody. (B) GST-GEF binds preferentially to GDP-bound RasB. Glutathione Sepharose beads coated with GST-GEF were incubated with AX2 cell lysates preincubated with 100 μM GTPγS or 100 μM GDP. Pulldown eluates were immunoblotted with RasB antibody. (C) RasB is activated upon starvation (6 h) and in cells overexpressing GFP-GEF (0 h), using beads coated with GST-Byr2(RBD). (D) Activation of RasB in response to cAMP. Aggregation-competent AX2 (top) cells were stimulated with 500 nM cAMP, and the amount of activated RasB bound to GST-Byr2(RBD) was determined at the indicated time points. Wild-type cells overexpressing GFP-DEP (bottom) showed reduced and delayed RasB activation upon cAMP stimulation. (E) Interaction between the DEP and the GEF domain of RasGEF Q. Glutathione Sepharose beads coated with GST-DEP and incubated with cell-free extracts from cells expressing GFP-GEF or GFP alone. (F) Deletion of DEP domain causes RasB activation. Vegetative cells overexpressing GFP-Δ-DEP-GEFQ and AX2 cells were used to pull down activated RasB using GST-Byr2–coated beads. Total cell lysates and pulldown eluates were immunoblotted with RasB antibody.