Fig. 7.
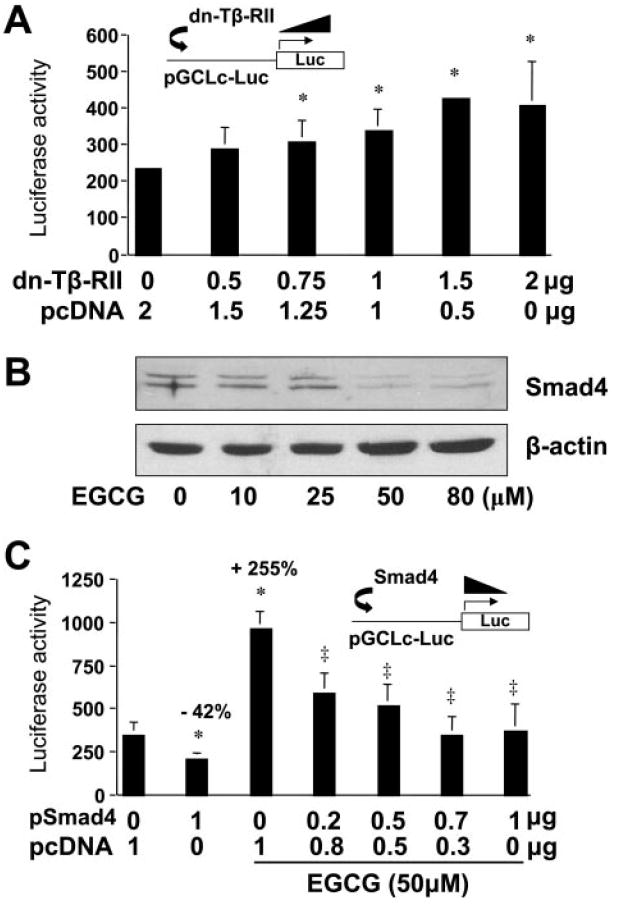
Interrupting TGF-β signaling by EGCG results in the induction of GCLc gene expression in activated HSCs in vitro. Passaged HSCs were cotransfected with the GCLc promoter luciferase reporter plasmid pGCLc-Luc and a cDNA expression plasmid, either pdn-TβRII, encoding the dominant-negative form of Tβ-RII (A), or pSmad4, encoding the constitutively active form of Smad4 (C). The empty vector pcDNA was used to ensure an equal amount of total DNA in transfection assays. Luciferase activities were normalized to β-galactosidase activity. Values are means ± S.D. (n = 3). *, p < 0.05 versus cells transfected with no pdn-Tβ-RII or pSmad4 (the first column on the left in A or C, respectively). ‡, p < 0.05 versus cells treated with EGCG without pSmad4 (the third column on the left in C). B, Western blotting analyses of the abundance of Smad4 in passaged HSCs treated with EGCG at various concentrations for 24 h. β-Actin was used as an internal control for equal loading. Representative was shown from three independent experiments.
