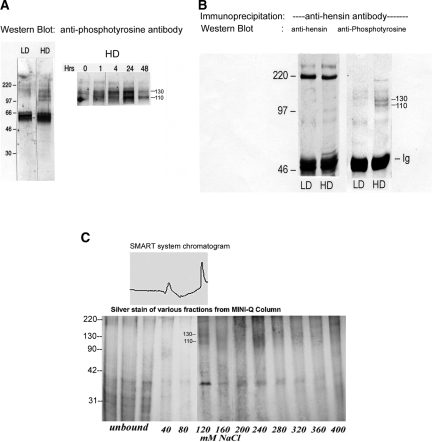
Figure 1.
Identification of basolateral membrane proteins that interact with hensin. (A) Equal amounts of basolateral membrane proteins from low- (LD) and high-density (HD) cells were immunoblotted with anti-phosphotyrosine antibody (left). Two prominent protein bands at 110 and 130 kD were visible only in the HD phenotypes but not in the LD phenotypes (left). These two bands were also seen in HD cell lysates purified by wheat germ agglutinin and probed with anti-phosphotyrosine antibody, indicating these are glycosylated proteins (right). Maximal phosphorylation of these two bands was observed after 24 h of seeding (right). (B) Chemical cross-linking of the basolateral surface followed by immunoprecipitation with hensin antibodies. Only samples from HD cells showed 110- and 130-kD bands when probed with anti-phosphotyrosine antibody. The blots were stripped and reprobed with anti-hensin antibody showing equal amounts of hensin in both LD and HD samples (220-kD band). (C) Glycosylated membrane proteins from HD cells, purified using a Wheat Germ Lectin column, were adsorbed onto Mini-Q resin ion-exchange column and eluted with salt. The 110-/130-kD tyrosine-phosphorylated protein bands eluted at approximately 120 mM NaCl (corresponding to the second peak in the top panel). These bands were excised and analyzed using MALDI-TOF.