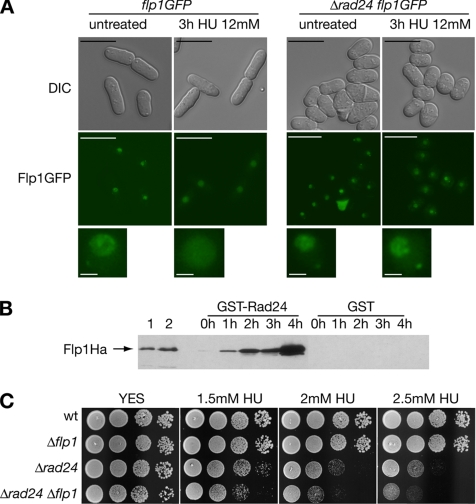
Figure 2.
Functional interaction between Rad24p and Flp1p in response to blocks in DNA replication. (A) Flp1p does not change its subcellular localization in response to replicative stress in Δrad24 cells. Live imaging of Flp1p-GFP in a strain deleted for rad24 after 3 h of 12 mM HU treatment. Flp1p remains nucleolar, whereas in control cells the staining is nuclear, as described previously. Bars, 10 μm. A 3× magnification of nuclei is shown in the bottom panel. Bars, 1 μm. (B) Rad24p interacts physically in vivo with Flp1p in checkpoint response to HU. GST-Rad24p was purified from untreated cells and at different times of 12 mM HU treatment. Blots were incubated with α-Ha antibody to detect Flp1pHa. Purified GST was used as a control. In lane 1, 10 μg of whole cell protein extract and in lane 2, 1/200 of the input protein in the pull-down assay shown for reference. No Rad24p–Flp1p interaction is detected in untreated cells, whereas interaction is detected at all treatment points analyzed. (C) Sensitivity assay of Δrad24 and Δrad24 Δflp1 cells to chronic HU exposure at 25°C. Δrad24 is partially sensitive to HU treatment. Δrad24 Δflp1 presents a more sensitive phenotype than each simple mutant.