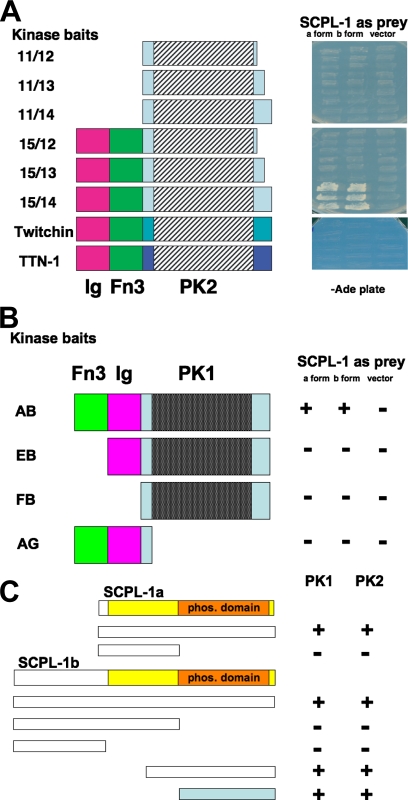
Figure 1.
(A) Yeast two-hybrid assays demonstrate the specificity of the interaction of UNC-89 PK2 with SCPL-1. Left, schematic representation of baits used to test full-length SCPL-1a and -b prey. Right, images of yeast growth on −Ade plates. Note that for UNC-89, interaction requires the catalytic core (PK2) plus the N-terminal Ig and Fn3 domains, and the C-terminal autoinhibitory domain. Note that comparable regions from the two other giant kinases in the worm, twitchin and TTN-1, fail to interact with SCPL-1. (B) The PK1 region of UNC-89 also interacts with SCPL-1 in the yeast two-hybrid assay. Domain mapping indicates that interaction of the PK1 region with SCPL-1 requires, in addition to the catalytic core (PK1), the Fn3 and Ig domains. +, growth and −, no growth on −Ade plates. (C) Only the phosphatase domain of SCPL-1 is required for interaction to the PK regions of UNC-89 in yeast two-hybrid assays. The indicated portions of SCPL-1a and -1b were tested as bait against Fn3-Ig-PK1 (PK1) or Ig-Fn3-PK2 (PK2) prey. +, growth and −, no growth on −Ade plates. The colored bar indicates the minimal region of SCPL-1a/b required for interaction.