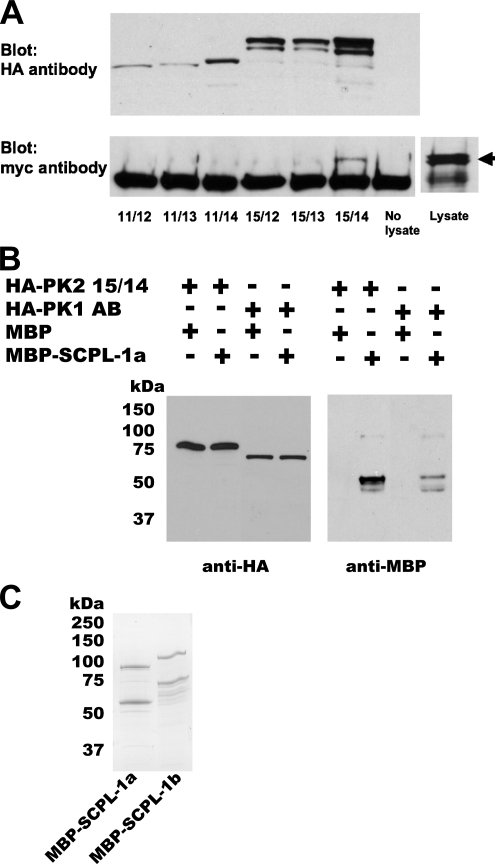
Figure 2.
Verification of interaction between SCPL-1 and UNC-89 PK1 or PK2 regions. (A) In the same yeast cell, myc-tagged SCPL-1b and individual HA-tagged derivatives of the UNC-89 PK2 region were expressed. 11/12, 11/13 … refer to the derivatives presented in Figure 1A. Total protein lysates were incubated with agarose beads coated with antibodies to the HA tag, washed, and eluted, and portions were run on two gels and blotted. One blot was reacted with anti-HA to detect the presence of the PK2 derivatives (top). As shown above, appropriately sized proteins were detected from each PK2 derivative. The other blot was reacted with anti-myc to detect the presence of SCPL-1 that might have been brought down with the PK2 protein (bottom). Only derivative 15/14 (Ig-Fn3-PK2-autoinhibitory region) is coimmunoprecipitated. An arrow designates the position on the blot of myc-SCPL-1b from the lysate. (B) Bacterially expressed MBP-SCPL-1a interacts with yeast expressed HA-PK1 or PK2. Total protein extracts were prepared from yeast expressing either HA-PK2 15/14 (see Figure 1A) or HA-PK1 AB (see Figure 1B), incubated with agarose beads coated with antibodies to HA, washed, incubated with purified, bacterially expressed MBP or MBP-SCPL-1a, and washed. The proteins were eluted, and portions of each sample were run on two gels and blotted. One blot was reacted with anti-HA to detect the presence of the yeast expressed protein, the other blot was reacted with anti-MBP to detect possible binding with MBP or MBP-SCPL-1a. (C) Coomassie stained gel of purified MBP-SCPL-1a and MBP-SCPL-1b. In each case, the top band is likely to be the full-length fusion protein; the second band may have resulted from degradation.