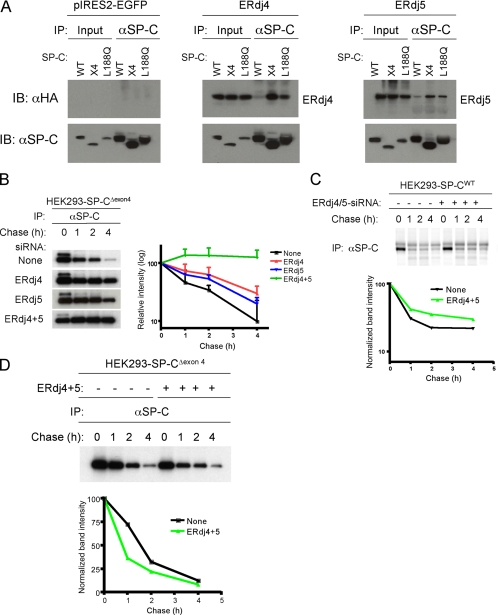
Figure 1.
ERdj4 and ERdj5 are required for degradation of SP-CΔexon4. (A) HEK293 cell lines stably expressing SP-CWT, SP-CΔexon4 (x4) or SP-CL188Q were transfected with plasmids encoding ERdj4 (middle), ERdj5 (right), or vector (left). Forty-eight hours after transfection, cells were treated with the proteasome inhibitor MG-132 to stabilize misfolded SP-C. After cross-linking with DSP, cells were harvested and equal amounts of cell lysates were subjected to IP with SP-C proprotein antibody followed by reducing SDS-PAGE, and sequential Western blotting (IB) with HA or SP-C antibodies. (B) HEK293 cells stably expressing SP-CΔexon4 were not transfected (top row) or transfected with siRNA for ERdj4, ERdj5, or both ERdj4 and ERdj5. Twenty-four hours later, cells were labeled with [35S]Met/Cys for 15 min, chased for the indicated period, and immunoprecipitated with SP-C antibody (left). The radioactivity of each SP-CΔexon4 band was analyzed by phosphorimaging analyses and normalized to the value at 0 chase time; averages of three independent experiments were plotted with SD values (right). (C) HEK293 cells stably expressing SP-CWT were transfected with siRNA for both ERdj4 and ERdj5 (lanes 5–8), or they were not transfected with siRNA (lanes 1–4). Pulse-chase, immunoprecipitation for SP-CWT proprotein, and phosphorimaging analyses were performed as described in B. (D) HEK293 cells stably expressing SP-CΔexon4 were cotransfected with plasmids encoding ERdj4 and ERdj5 (lanes 5–8), or they were left untransfected (lanes 1–4). Pulse-chase, immunoprecipitation, and phosphorimaging analyses were performed as described in B. The radioactivity of each SP-CΔexon4 band was normalized to the value at 0 chase time (bottom); pulse-chase studies represent the average of three independent experiments ± SD values.