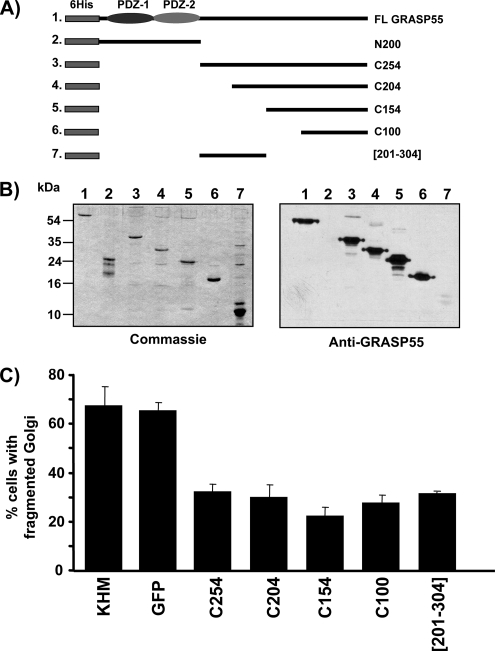
Figure 6.
The C-terminal domain of GRASP55 is required for Golgi fragmentation. (A) Schematic drawing of the recombinant full-length GRASP55 protein and the peptide fragments N200, C254, C204, C150, C100, and [201-304] used in this study. All constructs contain an N-terminal 6 histidine tag (6His). The N-terminal PDZ domains are shown. (B) Recombinant full-length GRASP55, the N-terminal fragment (N200 represents amino acids 1-200), C254 (amino acids 201-454), C204 (amino acids 251-354), C150 (amino acids 301-354), C100 (amino acids 351-454), and the internal peptide [201-304] (amino acids 201-304) were expressed in bacteria and purified on a nickel-agarose column. The recombinant proteins were analyzed by SDS-PAGE, and they were either Western blotted using the affinity-purified anti-GRASP55 antibody, or with Coomassie Blue to determine total protein levels. Anti-GRASP55 antibody recognizes only the C-terminal region of the protein. Note the low affinity of the antibody against the internal peptide [201-304] representing amino acids 201-304. (C) The recombinant C-terminal fragments C254 (amino acids 201-454), C204 (amino acids 251-354), C150 (amino acids 301-354), C100 (amino acids 351-454), [201-304] (amino acids 201-304), KHM buffer alone, or His-tagged GFP as a control were tested in the in vitro assay reconstituting Golgi fragmentation in NRK cells by mitotic cytosol. The percentage of cells with fragmented Golgi membranes was determined by immunofluorescence microscopy using an anti-giantin or anti-mannosidase II antibody. The data shows an average of four independent experiments per sample.