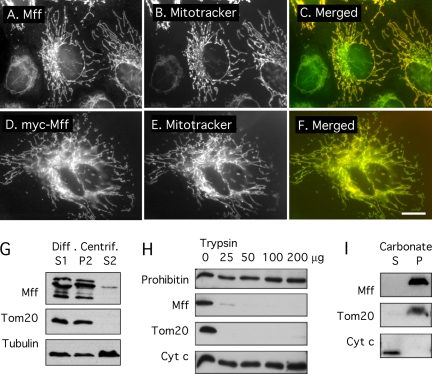
Figure 2.
Fluorescence and biochemical analysis of Mff localization. (A–C) Immunofluorescence of endogenous protein in HeLa cells. (D–F) Overexpression of myc-tagged Mff. The construct used for this experiment encodes isoform 8, which lacks exons 5, 6, and 7 (Figure 1). A and D show Mff or myc antibody staining, B and E show MitoTracker staining, and C and F show merged images with Mff or myc in green and MitoTracker in red. Bar, 10 μm. (G) Western blots of differential centrifugation fractions prepared from HeLa cells. Mff is present in the low speed supernatant (S1) and the medium speed pellet (P2), which contain mitochondria as shown with Tom20 antibody. Tubulin, which was used to track soluble proteins, is also present as a contaminant in the P2 fraction, but very little Mff is present in the S2 fraction, consistent with mitochondrial localization. The Mff antibody detects bands ranging in size from 25 to 39 kDa, most likely corresponding to different splice variants. (H) Protease protection experiment using the mitochondrial (P2) fraction from bovine brain to determine the topology of Mff. Our Mff antibody detects only a single band of 38-kDa in brain extracts, similar in size to the top band in HeLa cells (see G), suggesting that the repertoire of Mff isoforms is more limited in brain than it is in HeLa cells. The P2 fraction was subjected to increasing amounts of trypsin. Proteins that are exposed to the cytosol, like Tom20, are digested at the lowest concentrations, whereas proteins that are protected by membrane, such as the mitochondrial intermembrane space proteins prohibitin and cytochrome c are still protected at the highest concentration. Solubilization with detergents was used to verify that trypsin is able to digest prohibitin and cytochrome c were it not for protection by membrane (data not shown). Together, these data show that Mff is exposed to the cytosol. (I) Alkaline extraction shows that Mff is anchored in membrane. The P2 fraction from bovine brain extracts was resuspended in carbonate buffer, pH 11.5. Membranes were pelleted by centrifugation at 100,000 × g. Tom20 serves as marker for the membrane fraction, and cytochrome c as marker for the cytosolic fraction. These experiments show that Mff cofractionates with mitochondria, that it is exposed to the cytosol, and that it is a membrane-anchored protein.