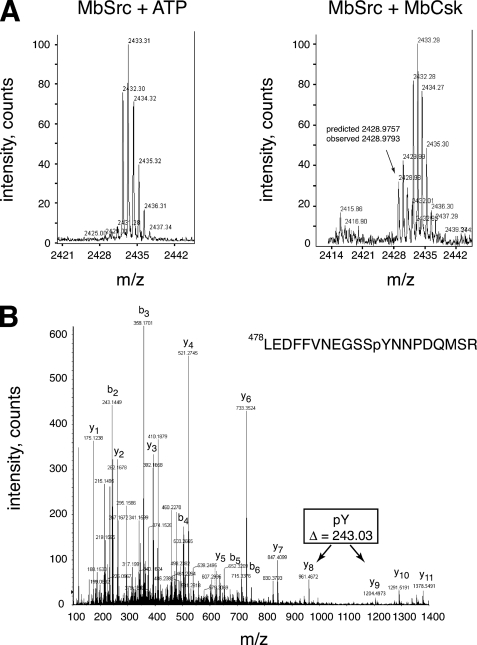
FIGURE 8.
Phosphorylation of the MbSrc1 C terminus by MbCsk. A, YOP-treated MbSrc1 was incubated in the presence or absence of MbCsk as described above for 45 min at 30 °C. Reactions were separated by SDS-PAGE, and gel bands corresponding to MbSrc1 were excised and subjected to in-gel tryptic digestion. The tryptic peptides for the control reaction (left) or MbCsk reaction (right) were analyzed by MALDI-TOF mass spectrometry. The position of the peak arising from the MbSrc1 C-terminal tail is indicated, with the predicted mass based on a phosphorylated sequence. This sequence is derived from residues Leu-478—Met-495 of MbSrc1, plus a C-terminal SR dipeptide derived from the baculovirus vector. Supplemental Fig. S7 presents MALDI-TOF data with an expanded m/z range. B, tandem mass spectrometric analysis of the C-terminal tryptic phosphopeptide from MbSrc1 shown in panel B. The b- and y-series of fragmentation ions are indicated. The difference in mass between the y8 and y9 ions indicate that the peptide is phosphorylated at the position corresponding to Tyr-489.