FIGURE 5.
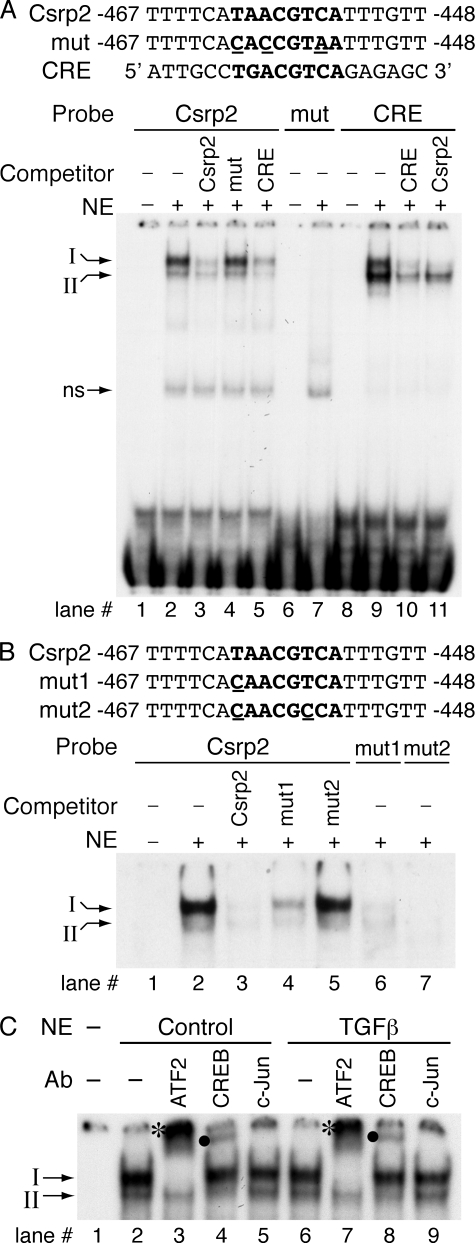
Nuclear proteins binding to the CRE-like element of Csrp2 promoter. A, oligonucleotide sequences used in the electrophoretic mobility shift assays (EMSAs) are shown. The core sequence of CRE-like and consensus CRE sites are in boldface type, and mutated sequence is underlined. EMSAs were performed with double-stranded oligonucleotides corresponding to bp –467 to –448 of the Csrp2 promoter. The addition of nuclear extracts (10 μg) from VSMCs to the 32P-labeled Csrp2 probe resulted in two major retarded DNA-protein complexes, designated I and II (arrows on left)(lane 2). A nonspecific band (ns) is indicated. Complexes I and II were abolished by the addition of unlabeled identical (Csrp2, lane 3) or consensus CRE (lane 5) oligonucleotides as competitors but not by the addition of three bases mutated (mut) oligonucleotides (lane 4). Conversely, EMSAs using 32P-labeled mutated oligonucleotides did not result in specific complex formation (lane 7). As a comparison, EMSAs using 32P-labeled CRE oligonucleotides were performed. The addition of nuclear extracts to the CRE probe resulted in the formation of complexes I and II (lane 9), which were competed away by identical unlabeled oligonucleotides (CRE; lane 10). The addition of unlabeled Csrp2 mainly abolished complex I and to a lesser degree complex II (lane 11). B, oligonucleotides used in the EMSAs are indicated. Csrp2 oligonucleotides contain the core sequence of CRE-like site (in boldface type), whereas mut1 has a one-base mutation (underlined) in the core and mut2 has two bases mutated (underlined). As in A, complex I and II were abolished by the addition of unlabeled identical (Csrp2, lane 3) oligonucleotides as competitors. mut1 partially competed away the complexes (lane 4), whereas mut2 did not compete away the complexes (lane 5). EMSAs using 32P-labeled mut1 oligonucleotides resulted in low intensity complex I and II formation (lane 6), whereas 32P-labeled mut2 oligonucleotides did not result in specific complex formation (lane 7). C, nuclear extracts from control (lanes 2–5) or TGFβ treated for 15 min (lanes 6–9) VSMCs were incubated with 32P-labeled Csrp2 probe without the addition of antibodies (lanes 2 and 6) or antibodies specific to ATF2 (lanes 3 and 7), CREB (lanes 4 and 8), or c-Jun (lanes 5 and 9). ATF2 antibody completely supershifted complex I to an upper band (*, lanes 3 and 7), whereas CREB antibody supershifted complex II to an upper complex (•, lanes 4 and 8). Incubation with c-Jun antibody did not produce supershifted bands (lanes 5 and 9).