Figure 1.
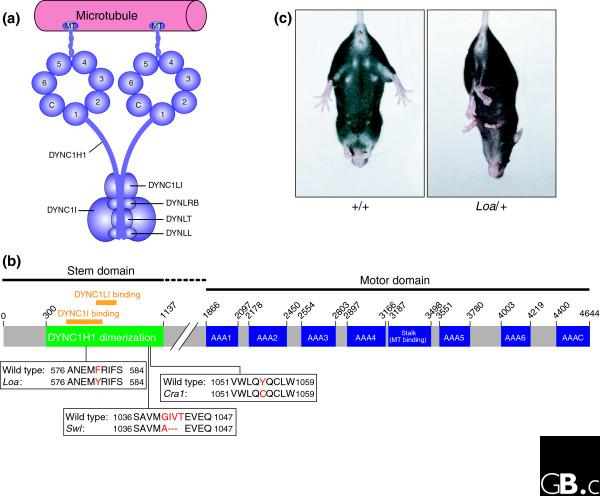
Heavy-chain dynein mutations. (a) A schematic diagram of the cytoplasmic dynein complex. The core of the complex comprises a homodimer of heavy-chain subunits (DYNC1H1), the carboxy-terminal half of which form seven AAA-ATPase domains (labelled 1 to 6 and C). The dynein intermediate (DYNC1I) and light-intermediate (DYNC1LI) chains bind to the amino-terminal domain of the heavy chains. The light chains (DYNLRB, DYNLT and DYNLL) all bind to the intermediate chains. The dynactin complex (not shown) binds to the cytoplasmic dynein intermediate chains. Adapted from [2]. (b) Protein domain map of the cytoplasmic dynein heavy chain, showing the location of the mutations Loa, Cra1 and Swl. The motor domain consists of the six known AAA-ATPase domains (AAA 1 to 6) and an unrelated seventh domain (AAAC). The microtubule-binding domain lies between AAA4 and AAA5. The amino-terminal half of the protein contains the intermediate (DYNC1I), light-intermediate (DYNC1LI) and heavy (DYNC1H1) chain binding domains [21,22]. The Loa mutation falls within both the DYNC1H1 dimerization and DYNC1I binding domains. The Cra1 and Swl mutations fall outside of the DYNC1I binding domain, but still within the DYNC1H1 dimerization domain. (c) The hind-limb clasping phenotype of Loa/+ mice. When held by the tail, wild-type (+/+) mice splay their hind legs away from their body. In contrast, Loa/+ mice withdraw their hind limbs, pulling them into their body. Swl/+ mice display a similar phenotype.
