Fig. 1.
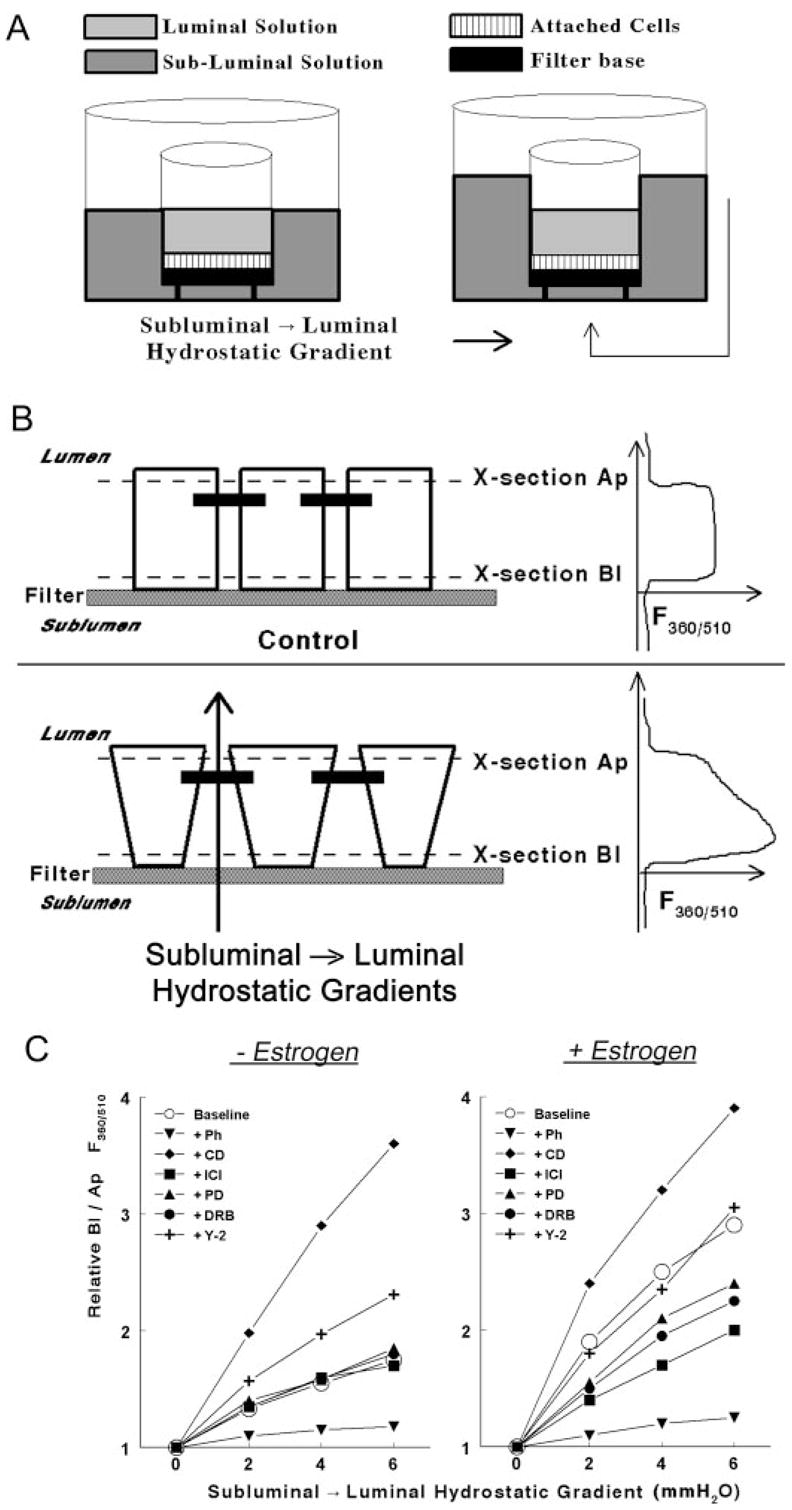
Methodology to determine rigidity of the cytoskeleton in epithelial cells attached on filters. A, A filter insert with cells (squares) attached on the filter base (filled square) was placed in a fluorescence chamber in which the luminal (light gray) and subluminal (darker gray) solutions could be independently perfused. subluminal → luminal hydrostatic gradients were established by increasing the volume of the subluminal solution above that of the luminal solution. B, Schema and cross-section of epithelial cells attached on filters. Neighboring cells are joined by junctional and adherens junctions that localize near the apical border of the cells (filled horizontal bars). In the lower panel, hydrostatic gradients in the subluminal → luminal direction were established as described above. The panels in the right parts of B are actual recordings of the F360/510 along the longitudinal vertical (Z)-axis of the cultures. See Materials and Methods for details. C, Modulation of the Bl to Ap F360/510 ratio in hEVECs attached on filters by subluminal → luminal hydrostatic gradients. Cells were shifted for 3 d to steroid free medium and treated for 2 d before the experiment with the vehicle (− Estrogen) or 10 nM 17β-estradiol (+ Estrogen). Additional cotreatments were as follows: phalloidin (Ph, 10 ng/ml, 60 min); DRB (10 μM, 6 h); Y-27632 (Y-2, 5 μM, 6 h); cytocha-lasin-D (CD, 10 μg/ml, 20 min); ICI-182,780 (ICI, 10 μM, 1 d); and PD98059 (PD, 10 μM, 6 h). Each line represents means of three to four experiments. Data were normalized to maximal increases of the Bl to Ap F360/510 ratio (arbitrary value of 4) as determined in cells treated with cytochalasin-D. Variability ranged 3–7%. Similar experiments were done using CaSki cells (not shown).
