Abstract
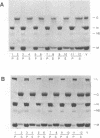
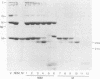
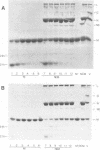
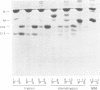
The purpose of these experiments was to study the physical structure of the nucleocapsid-M protein complex of vesicular stomatitis virus by analysis of nucleocapsid binding by wild-type and mutant M proteins and by limited proteolysis. We used the temperature-sensitive M protein mutant tsO23 and six temperature-stable revertants of tsO23 to test the effect of sequence changes on M protein binding to the nucleocapsid as a function of NaCl concentration. The results showed that M proteins from wild-type, mutant, and three of the revertant viruses had similar NaCl titration curves, while the curve for M proteins from the other three revertants differed significantly. The altered NaCl dependence of M protein was correlated with a single amino acid substitution from Phe to Leu at position 111 compared with the original temperature-sensitive mutant and was not correlated with a substitution of Gly to Glu at position 21 in tsO23 and the revertants. To determine whether protease cleavage sites in the M protein were protected by interaction with the nucleocapsid, nucleocapsid-M protein complexes were subjected to limited proteolysis with trypsin, chymotrypsin, or Staphylococcus aureus V8 protease. The initial trypsin and chymotrypsin cleavage sites, located after amino acids 19 and 20, respectively, were as accessible to proteases when M protein was bound to the nucleocapsid as when it was purified, indicating that this region of the protein does not interact directly with the nucleocapsid. Furthermore, trypsin or chymotrypsin treatment released the M protein fragments from the nucleocapsid, presumably due to conformational changes following proteolysis. V8 protease cleaved the M protein at position 34 or 50, producing two distinct fragments. The M protein fragment produced by V8 protease cleavage at position 34 remained associated with the nucleocapsid, while the fragment produced by cleavage at position 50 was released from the nucleocapsid. These results suggest that the amino-terminal region of the M protein around amino acid 20 does not interact directly with the nucleocapsid and that conformational changes resulting from single-amino-acid substitutions at other sites in the M protein are important for this interaction.
Full text
PDF

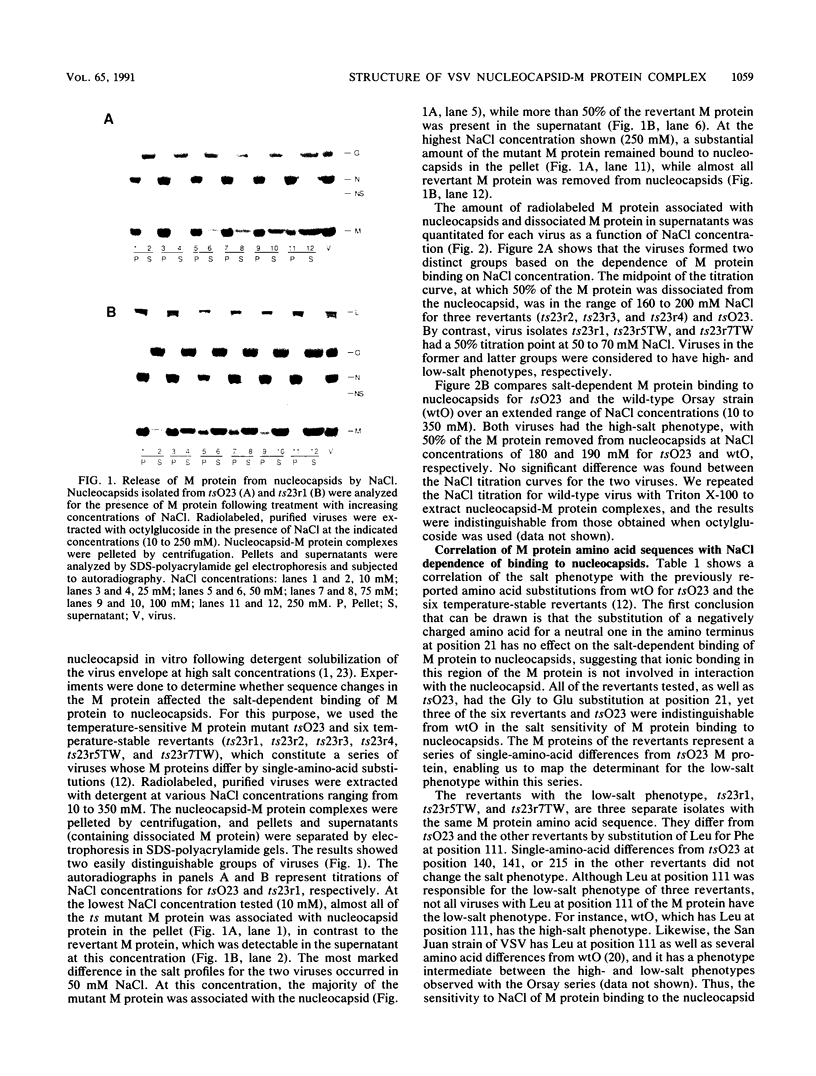
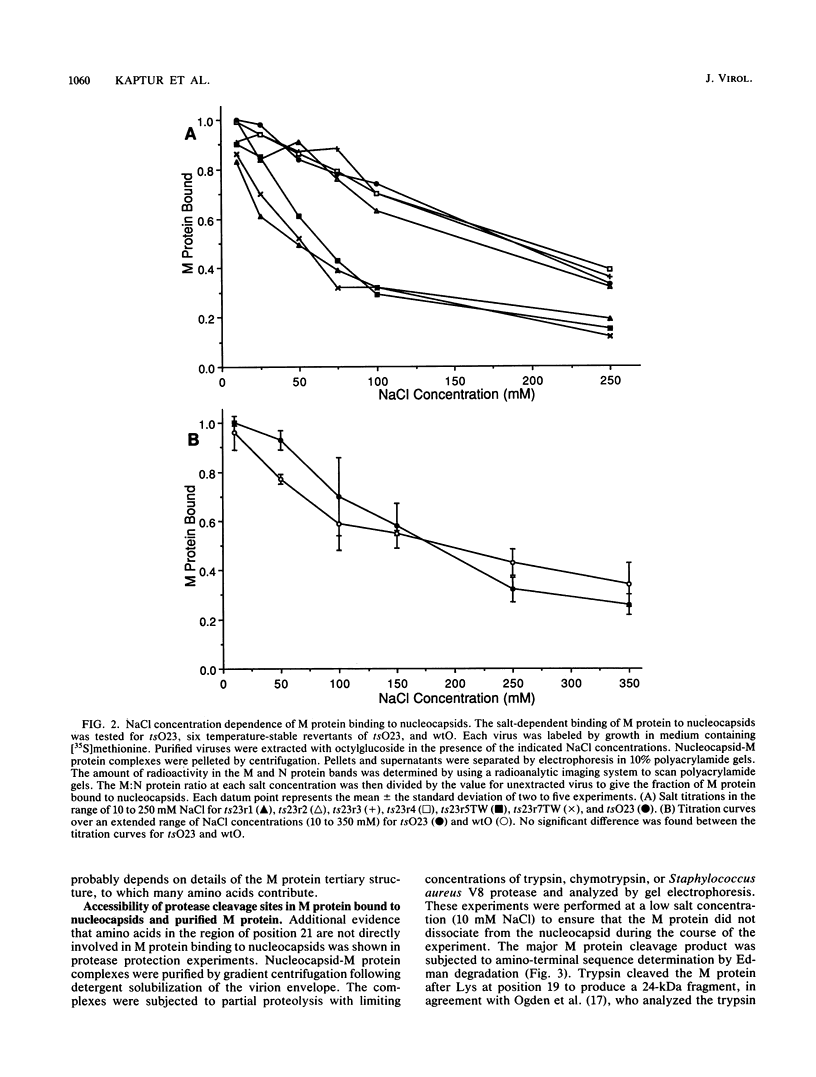
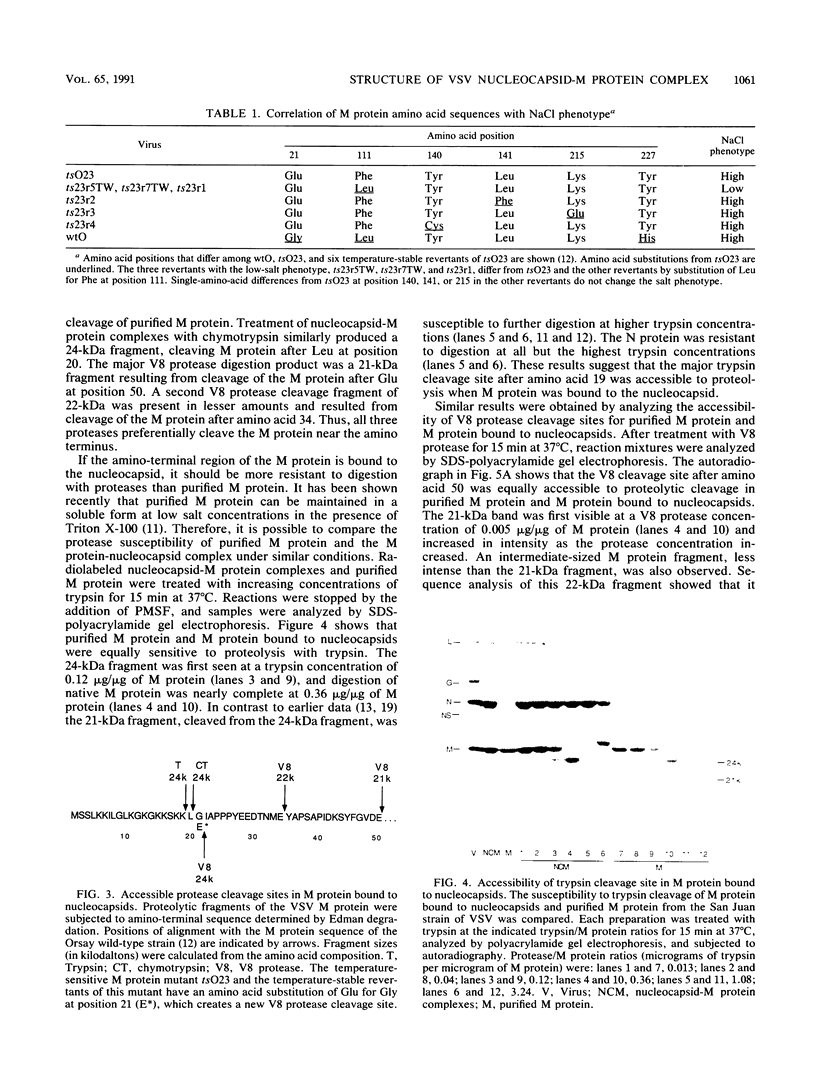



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J., Vanderoef R. Localization of the membrane-associated region of vesicular stomatitis virus M protein at the N terminus, using the hydrophobic, photoreactive probe 125I-TID. J Virol. 1990 Jul;64(7):3486–3491. doi: 10.1128/jvi.64.7.3486-3491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Site-specific mutations in vectors that express antigenic and temperature-sensitive phenotypes of the M gene of vesicular stomatitis virus. J Virol. 1988 Oct;62(10):3729–3737. doi: 10.1128/jvi.62.10.3729-3737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Wagner R. R. Transcription inhibition site on the M protein of vesicular stomatitis virus located by marker rescue of mutant tsO23(III) with M-gene expression vectors. J Virol. 1989 Jun;63(6):2841–2843. doi: 10.1128/jvi.63.6.2841-2843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McCreedy B. J., Jr, McKinnon K. P., Lyles D. S. Solubility of vesicular stomatitis virus M protein in the cytosol of infected cells or isolated from virions. J Virol. 1990 Feb;64(2):902–906. doi: 10.1128/jvi.64.2.902-906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Vanderoef R., Lenard J. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J Virol. 1987 Feb;61(2):256–263. doi: 10.1128/jvi.61.2.256-263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. O. Assembly of viral membranes: nature of the association of vesicular stomatitis virus proteins to membranes. J Virol. 1978 Apr;26(1):115–125. doi: 10.1128/jvi.26.1.115-125.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J. B., Pal R., Wagner R. R. Antigenicity, function, and conformation of synthetic oligopeptides corresponding to amino-terminal sequences of wild-type and mutant matrix proteins of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2569–2577. doi: 10.1128/jvi.62.8.2569-2577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]