Abstract
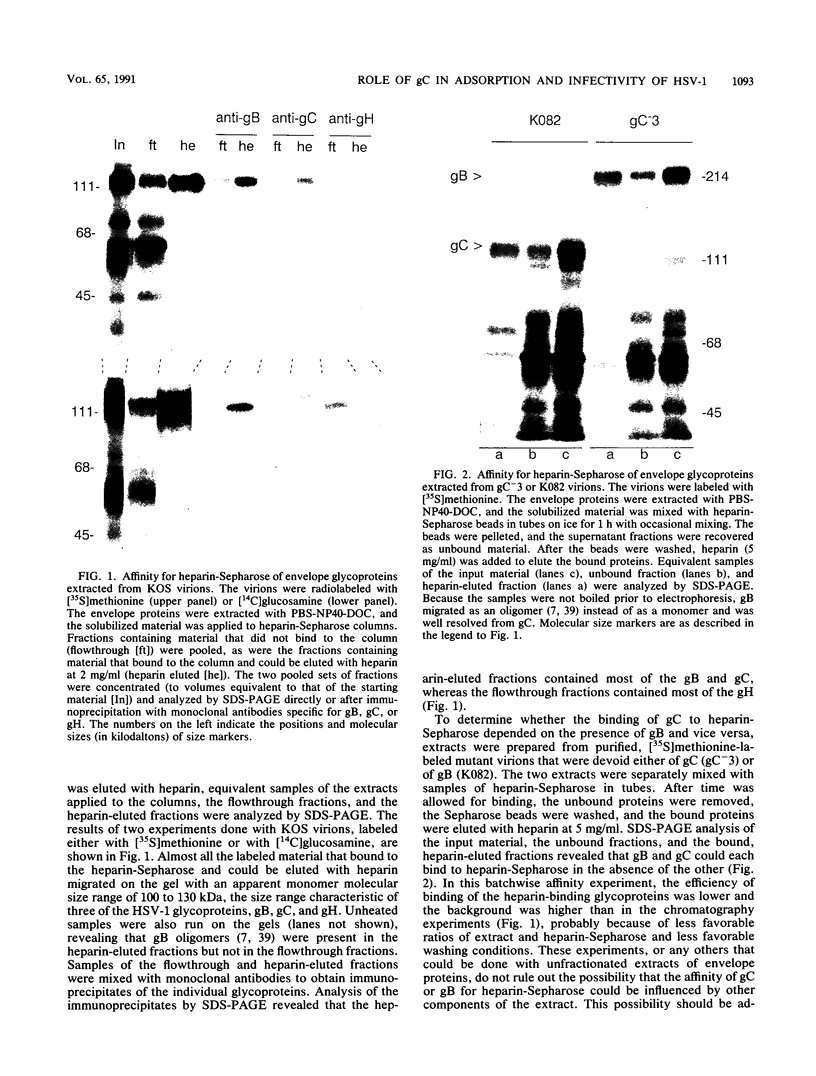
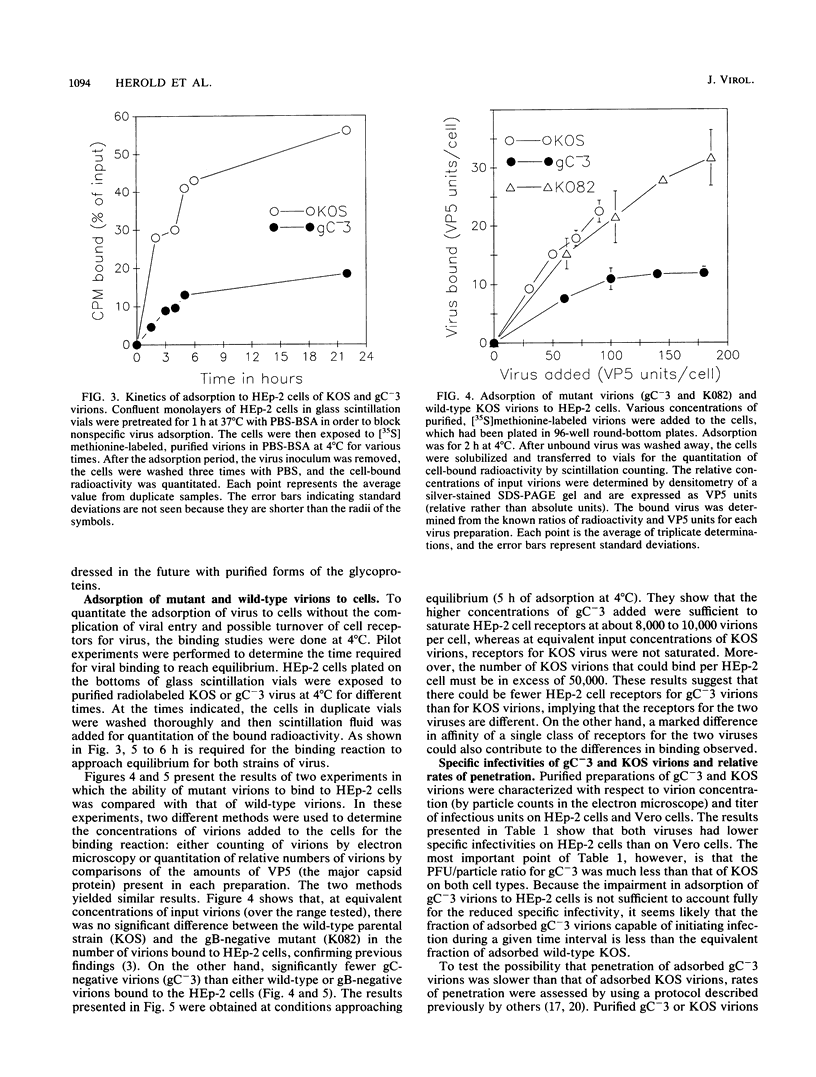
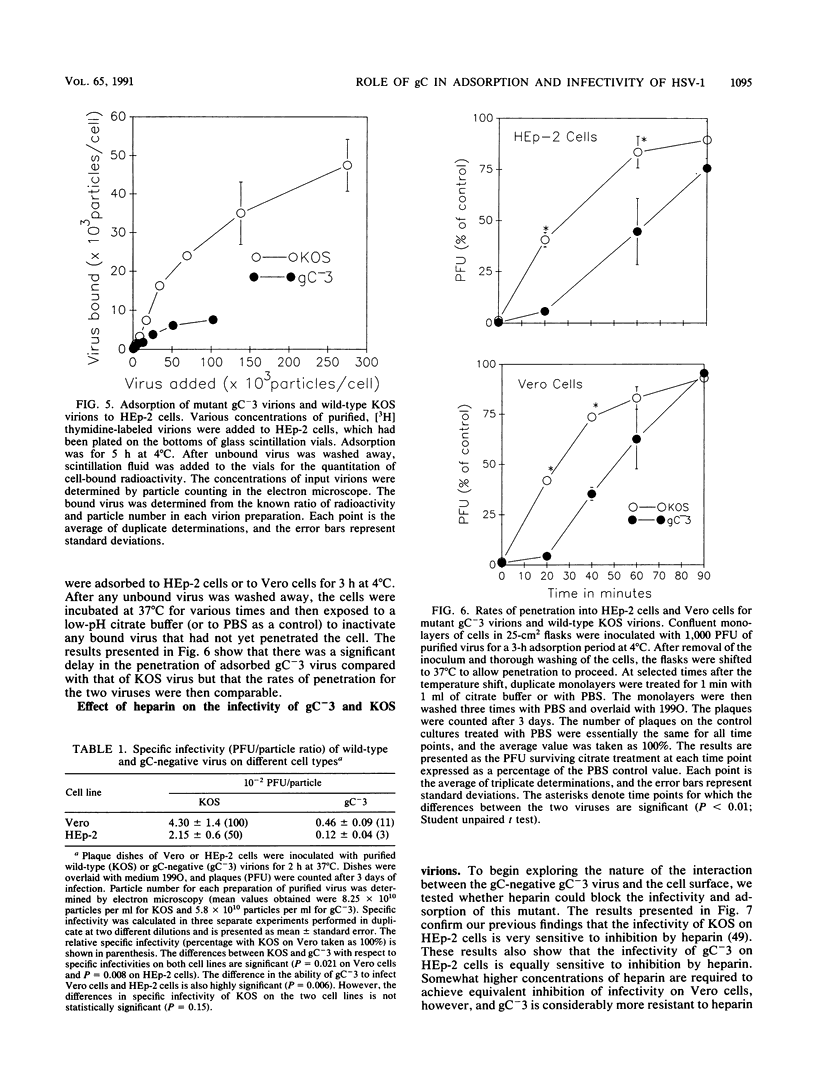
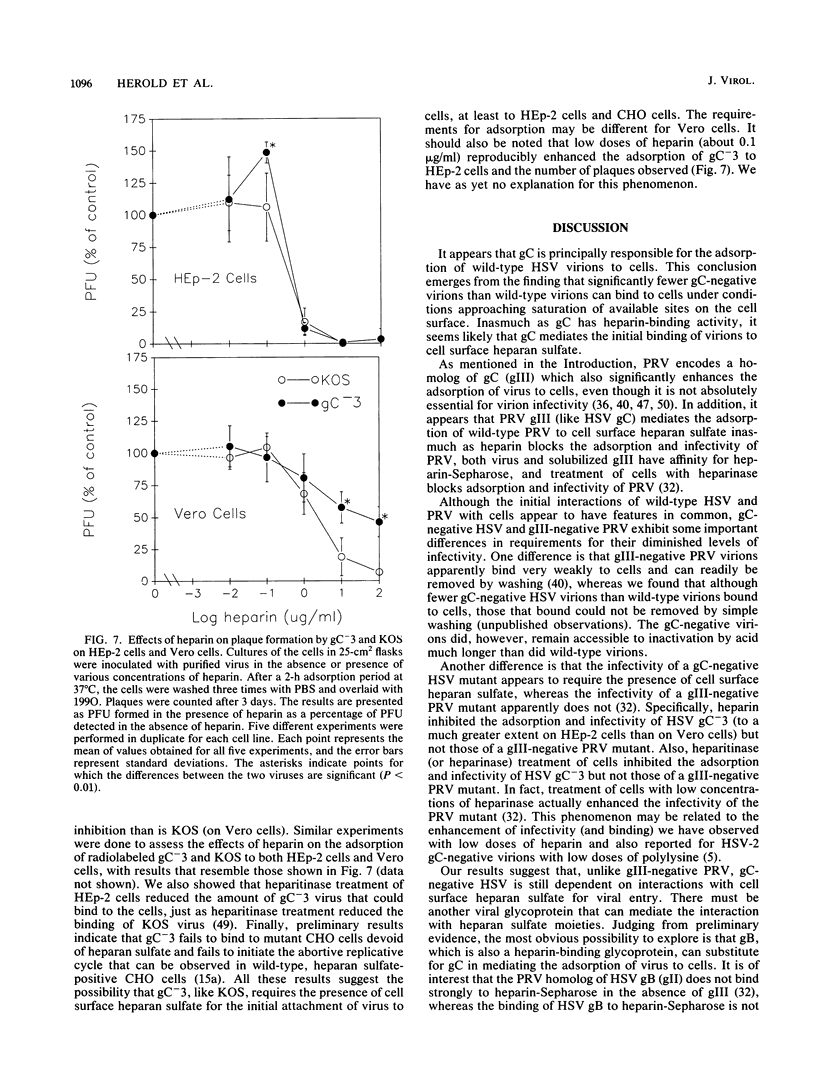
The purpose of this study was to identify the herpes simplex virus glycoprotein(s) that mediates the adsorption of virions to cells. Because heparan sulfate moieties of cell surface proteoglycans serve as the receptors for herpes simplex virus adsorption, we tested whether any of the viral glycoproteins could bind to heparin-Sepharose in affinity chromatography experiments. Two glycoproteins, gB and gC, bound to heparin-Sepharose and could be eluted with soluble heparin. In order to determine whether virions devoid of gC or gB were impaired for adsorption, we quantitated the binding of wild-type and mutant virions to cells. We found that at equivalent input concentrations of purified virions, significantly fewer gC-negative virions bound to cells than did wild-type or gB-negative virions. In addition, the gC-negative virions that bound to cells showed a significant delay in penetration compared with wild-type virus. The impairments in adsorption and penetration of the gC-negative virions can account for their reduced PFU/particle ratios, which were found to be about 5 to 10% that of wild-type virions, depending on the host cell. Although gC is dispensable for replication of herpes simplex virus in cell culture, it clearly facilitates virion adsorption and enhances infectivity by about a factor of 10.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Person S., Warner S. C., Zhou J. H., DeLuca N. A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987 Mar;61(3):714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Avitabile E., Fini S., Stirpe D., Arsenakis M., Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988 Oct;166(2):598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Stirpe D., Boscaro A., Avitabile E., Foá-Tomasi L., Barker D., Roizman B. Glycoprotein C-dependent attachment of herpes simplex virus to susceptible cells leading to productive infection. Virology. 1990 Sep;178(1):213–222. doi: 10.1016/0042-6822(90)90396-9. [DOI] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Spear P. G. Oligomerization of herpes simplex virus glycoprotein B. J Virol. 1986 Nov;60(2):803–806. doi: 10.1128/jvi.60.2.803-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner R. J., Baird A., Mansukhani A., Basilico C., Summers B. D., Florkiewicz R. Z., Hajjar D. P. Fibroblast growth factor receptor is a portal of cellular entry for herpes simplex virus type 1. Science. 1990 Jun 15;248(4961):1410–1413. doi: 10.1126/science.2162560. [DOI] [PubMed] [Google Scholar]
- Langeland N., Holmsen H., Lillehaug J. R., Haarr L. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to the cellular receptor. J Virol. 1987 Nov;61(11):3388–3393. doi: 10.1128/jvi.61.11.3388-3393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeland N., Moore L. J., Holmsen H., Haarr L. Interaction of polylysine with the cellular receptor for herpes simplex virus type 1. J Gen Virol. 1988 Jun;69(Pt 6):1137–1145. doi: 10.1099/0022-1317-69-6-1137. [DOI] [PubMed] [Google Scholar]
- Langeland N., Oyan A. M., Marsden H. S., Cross A., Glorioso J. C., Moore L. J., Haarr L. Localization on the herpes simplex virus type 1 genome of a region encoding proteins involved in adsorption to the cellular receptor. J Virol. 1990 Mar;64(3):1271–1277. doi: 10.1128/jvi.64.3.1271-1277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Mansukhani A., Moscatelli D., Talarico D., Levytska V., Basilico C. A murine fibroblast growth factor (FGF) receptor expressed in CHO cells is activated by basic FGF and Kaposi FGF. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard L. M., Fuller A. O., Spear P. G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987 Mar;68(Pt 3):715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus glycoprotein gIII is required for efficient virus growth in tissue culture. J Virol. 1988 Jul;62(7):2512–2515. doi: 10.1128/jvi.62.7.2512-2515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]