Abstract
Background
Sugar moieties of gonadotropins play no primary role in receptor binding but they strongly affect their circulatory half-life and consequently their in vivo biopotencies. In order to relate more precisely hepatic trapping of these glycoproteic hormones with their circulatory half-life, we undertook a comparative study of the distribution and elimination of porcine LH (pLH) and equine CG (eCG) which exhibit respectively a short and a long half-life. This was done first by following half-lives of pLH in piglets with hepatic portal circulation shunted or not. It was expected that such a shunt would enhance the short half-life of pLH. Subsequently, scintigraphic imaging of both 123I-pLH and 123I-eCG was performed in intact rats to compare their routes and rates of distribution and elimination.
Methods
Native pLH or eCG was injected to normal piglets and pLH was tested in liver-shunted anæsthetized piglet. Blood samples were recovered sequentially over one hour time and the hormone concentrations were determined by a specific ELISA method. Scintigraphic imaging of 123I-pLH and 123I-eCG was performed in rats using a OPTI-CGR gamma camera.
Results
In liver-shunted piglets, the half-life of pLH was found to be as short as in intact piglets (5 min). In the rat, the half-life of pLH was also found to be very short (3–6 min) and 123I-pLH was found to accumulate in high quantity in less than 10 min post injection at the level of kidneys but not in the liver. 123I-eCG didn't accumulate in any organ in the rats during the first hour, plasma concentrations of this gonadotropin being still elevated (80%) at this time.
Conclusion
In both the porcine and rat species, the liver is not responsible for the rapid elimination of pLH from the circulation compared to eCG. Our scintigraphic experiments suggest that the very short circulatory half-life of LH is due to rapid renal trapping.
Background
Glycoprotein hormones (Luteinizing Hormone LH, Follicle-Stimulating Hormone FSH, Chorionic Gonadotropin CG and Thyroid-Stimulating Hormone TSH) are comprised of two dissimilar, non-covalently bound glycoproteic subunits, named α and β and their 1:1 association is mandatory for hormonal activity. The α-subunit is common to all of them and it is encoded by an unique gene whereas the different β-subunits confer hormonal specificity to each αβ heterodimer.
It has been known for a long time that the sugar moieties of gonadotropins play no primary role in receptor binding affinity or specificity but that they strongly affect their circulatory half-life and consequently their in vivo biopotencies. Indeed, when completely deglycosylated, glycoprotein hormones exhibit neither in vivo nor in vitro bioactivity but they retain their ability to specifically bind to their receptors and so act as antagonists [1]. Carbohydrate moieties of glycoprotein hormones are thus not required for binding to their cognate receptors but they play a direct or indirect role in signal transduction. Desialylation of hCG, eCG or FSH leads to a dramatic decrease in their in vivo bioactivity, whereas in vitro bioactivity is unchanged or even increased [2]. Therefore, the terminal sialic acid residues of these hormones are important for their survival in circulation and desialylation which leads to a drop in their circulatory half-life also results in an almost complete loss of in vivo bioactivity.
If one aims at increasing the in vivo activity of glycoprotein hormones, it is essential to understand the mechanisms of removal of these hormones from the circulation and to identify the structural features implicated in that process. It is generally postulated that desialylated hormones having galactose exposed after sialic acid removal are captured by the liver by binding to the membrane galactose receptor of Ashwell [3], and that LH possessing sulfated terminal residues are extracted from blood flow by hepatic reticuloendothelial sulfated-GalNAc receptor of Baezinger [4]. In keeping with this view, pituitary LH from different species which are poorly or not sialylated at all, exhibit very short half-life compared to FSH and even more compared to CG.
Nevertheless, studies from several groups in the seventies suggested that kidneys could be implicated in gonadotropin removal from blood. Indeed, Gay [5] and De Kretser et al [6] showed that upon nephrectomy, half-life of LH and FSH were increased. In addition, after injection of 3H-ovine LH, Ascoli et al [7] found that the radioactivity was mainly recovered in kidneys and urine.
Therefore, in order to get a better understanding of the mechanism of gonadotropin removal from circulation, we undertook a comparative pharmacokinetic study of pLH and eCG. Porcine LH was chosen because of its previously recognized very short half-life (only a few minutes) [7] while the well known long half-life of eCG (6 hours to 1 day or more, depending on the species and methods used) [8,9] has been exploited for more than half a century in the induction of follicular growth in domestic animals.
We first compared the plasma kinetics of the two hormones in piglets, and confirmed the short half-life of pLH in its own species. Subsequently, we studied the influence of a shunt of the hepatic portal vein on the half-life of porcine LH. Finally, in order to explore directly the distribution/elimination routes of gonadotropins, we undertook a scintigraphic imaging study with the two 123I-labelled hormones. We chose the rat as animal model, in order to get whole body imaging with the gamma camera used. To our knowledge, this is the first 123I scintigraphic imaging study of gonadotropin pharmacokinetics.
Methods
Animals
Male Meishan piglets, around 15 kg, were bred in the experimental pigsty of the INRA Centre in Nouzilly. Normal adult male and female Wistar male rats aged 52 days, mean weight 200 g, were obtained from the Small Animal Breeding Facility of the Laboratory.
All the procedures used in the experiments presented in this paper were in compliance with the European Community Council Directive of November 24 1986 (86/609/EEC).
pLH and eCG plasma kinetics in normal piglets
Piglets were halothane-anæsthetized before jugular and carotid catheterization. Blood samples were removed via the jugular cannula just before and every 5 min over a 1 h period after administration through the carotid of 1,5 mg of either pLH or eCG in saline. These samples were centrifuged 10 minutes at 3000 g at 4°C and the hormones concentrations were determined in plasma by specific competition enzyme-linked immunosorbent assays (ELISA) for eCG [10] and pLH [Lecompte F and Combarnous Y; unpublished]. Briefly, the pLH competitive ELISA was set-up using pLH from Dr Hennen's Laboratory [11] and rabbit anti-pLH serum (ref 61977) kindly given by Dr Jean Pelletier (INRA, Nouzilly). This antibody has been shown to be specific for LH in RIA with less than 1% cross-reaction with FSH [12]. The microtiter plates were coated with 10 ng pLH/100 μl at pH 9.6 overnight at 4°C and after washing, 50 μl of samples (reference or unknown) were added together with 50 μl antiserum diluted 1/80,000. After 4 hours at 4°C, the wells were emptied and washed five times and 100 μl peroxidase-conjugated anti-rabbit serum (BioRad, Marnes-la-Coquette, France) were added. After 1 hour at 4°C, the wells were emptied and washed three times and 100 μl of SureBlue™ substrate (KPL, Gaithersburg, Md, USA) were added and incubation was carried out for 30 minutes at room temperature in the dark. Finally, the reaction was stopped with 50 μl H2SO4 and the optical densities of each well at 450 nm were recorded on a SpectraCount spectrophotometer (Packard, Chicago, Il USA) and concentrations were calculated using the Packard RIASmart™ package.
Pharmacokinetic data were analyzed by non-linear fitting to exponential decay equation using Graph-Pad Prism™ version 2.0 package (Intuitive Software for Science, San Diego USA).
Kinetics of pLH in liver-shunted piglets
An halothane-anæsthetized piglet was catheterized in the right jugular vein and the hepatic portal vein and the cava vein were also catheterized and connected via a Y-tube to the jugular vein catheter so that veinous circulation to the liver is totally shunted. The hepatic vascular pedicle was also clamped, so that no more vascular exchanges with liver was possible. Hormone injection, blood collection, ELISAs and analyses were performed as described above.
Kinetics of pLH and eCG in normal rats
Five anaesthetized male rats received 20 μg pLH in the tail vein and four others received 20 μg eCG. Blood was withdrawn on heparin from the jugular vein. The samples were treated as previously described. The animals injected with pLH were sacrificed and urine aspirated from the bladder one hour post-injection. The animals injected with eCG were placed in a metabolic cage between two blood withdrawals in order to collect emitted urine and if no urine could be collected in this way, aspiration from bladder was performed as above. Hormonal concentrations in urine were determinated by ELISA as above.
Glycoprotein Hormones radiolabeling
Porcine LH (pLH DKL-D110; 3.0 × NIH-LH-P1) and eCG (eCG FL652; 6700 IU/mg) were purified in our laboratory [13,14] and 10 mg/ml stock solutions were prepared in 50 mM phosphate buffer pH 7.4. Radioiodination with 123I was performed using the Iodo-Gen® method [15] using [123I] sodium iodide (71.4 TBq/mg i.e. 8.78 × 1018 Bq/mol iodide; no carrier added) from Cis-bio international (Gif-sur-Yvette, France). Briefly, 96–110 MBq 123I in 100 μl 50 mM phosphate buffer pH = 7.4 were added to an Eppendorf tube precoated with 20 μg Iodo-Gen® (1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril, Pierce). Ten μl (100 μg, 2.27 × 10-9 mole eCG or 3.57 × 10-9 mole pLH) were then added to this tube, and the reaction was allowed to proceed for 10 min on ice. Then, the mixture was applied onto a sephadex G-25 column (PD-10, Pharmacia, Uppsala, Sweden) pretreated with the corresponding hormone to saturate non-specific sites, and eluted with saline solution to separate the labeled hormone from unconjugated 123I. The labeling efficiencies were 26–28% and after removal of free iodide, the final specific activities for the two hormones were approximately 0,3 MBq/μg which are similar to those reached for 123I-IL-8 [16]. The specific activities correspond to I/protein molar ratios of 1/1104 for 123I-pLH and 1/738 for 123I-eCG.
Biodistribution of radiolabeled hormones by gamma camera imaging
The rats were anaesthetized by halothane inhalation (1–3% in air influx at 1 l/min), placed prone on the gamma camera, and then immediately received a single IV injection of either 123I-pLH (2 rats), 123I-eCG (3 rats) or free 123I Na (3 rats).
After IV injection, dynamic 1-minute images were recorded over the first 30 minutes and 2-minute images after 1, 2, 3, 4, 5 and 6 hours for both pLH and eCG. In the case of eCG, images were also recorded after 7, 8, 9 and 10 hours. The animals were returned to their cage after each scintigraphy, and re-immobilized by slight halothane anæsthesia for the following one.
Images were recorded using a gamma OPTI-CGR camera equipped with low-energy high resolution parallel collimator. The energy peak was centred at 158 KeV (123I energy peak) with a 15% window. Planar images were acquired into 128 × 128 pixels matrix with a dedicated computer system for digital display and analysis (TIM 512; Motorola). After acquisition of 50000–100000 counts, the images were digitally stored in a 128 × 128 matrix. The scintigraphic results were analysed quantitatively by drawing regions of interest (ROI) created for heart, kidney, liver, and all body. The count rates recorded within these regions were used after correction for radioactive decrease, to calculate the percentage of radioactive hormone in each organ. One or two flasks with known 123I doses were placed beside the animal for camera calibration.
Half-life of 123I-pLH was determined by following emission at heart level.
Results
Kinetics of pLH and eCG in normal piglets
As shown on figure 1, plasma pLH concentrations measured by EIA decreased rapidly after injection in the piglet. On the contrary, 80% plasma eCG was still present one hour p.i. The half-life of pLH was found to be 4.50 min (95%CL 3.68–5.81).
Figure 1.
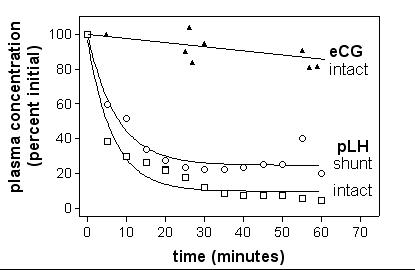
Half-life of porcine LH in intact and liver-shunted piglets by ELISA. Hepatic portal vein and vena cava of anesthetized female piglets were derived towards jugular vein as to completely suppress removal of gonadotropins from circulation by the liver. Control animals were sham-operated. Blood samples were continuously taken from contralateral jugular vein before and over 1 hour after injection. Hormone concentrations were measured by ELISA. Half-life of pLH in the two animal models was determined by non-linear regression of relative intensities as a function of time.
Kinetics of pLH in liver-shunted piglets
As illustrated in figure 1, plasma pLH elimination is not significantly affected when the hepatic veinous circulation is shunted towards jugular vein. The half-life of pLH was found to be 5.19 min (95%CL 3.70–8.64) and as in intact animals, 80 % plasma eCG was still present one hour p.i. This suggests a very limited role of the liver if any in the fast elimination of porcine LH from circulation.
Kinetics of pLH and eCG in normal rats
Kinetics of pLH elimination in the rat after IV injection was quite similar to that observed in the piglet. The half-life of pLH was found to be 6.17 min (95%CL 5.20–7.58). For eCG, plasma concentrations were still elevated one hour p.i. both in the rat (80%, figure 2) and the piglet (85%, figure 1).
Figure 2.
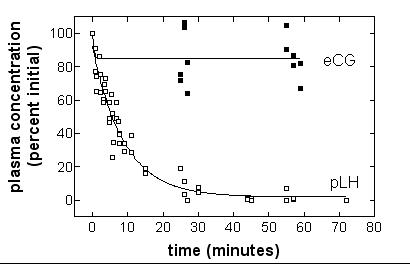
Half-life of porcine LH and equine CG in rats by ELISA. Halothane-anaesthetized rats aged 52 days, received 20 μg pLH or eCG in the tail vein. Blood was withdrawn on heparin from the jugular vein. Hormone concentrations were measured by ELISA. Half-life of pLH was determined by non-linear regression of concentrations as a function of time.
Hormonal recovery of pLH in urine one hour after injection was very low: only 1.2 to 2.5 ng pLH (less than 0,012% of the injected dose) were recovered in urine as measured by ELISA. In the case of eCG, 86 ng and 332 ng were recovered for two animals tested, which represented 0.4% and 1.7% of the injected dose. Therefore, only a tiny amount of gonadotropin was recovered in urine over a 1-hour period of time after injection whatever its half-life in circulation is short (pLH) or long (eCG).
Biodistribution of radiolabeled hormones by gamma camera imaging
Free 123Iodine
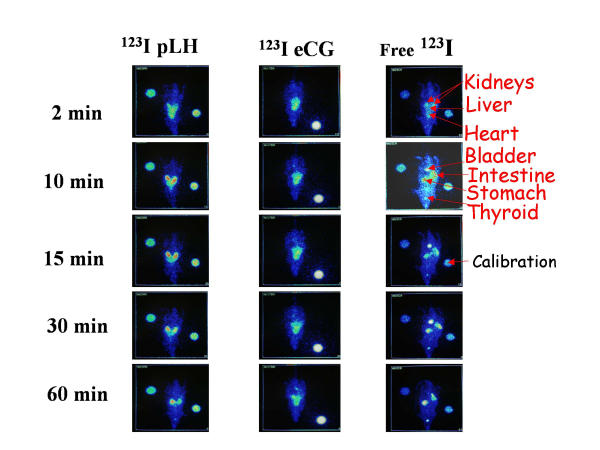
After IV injection, free iodine was observed in the vascular bed, as shown by the visualization of lungs and heart (figure 3; right panel). It is rapidly extracted (15 minutes p.i.) by stomach, intestine and thyroid gland. The radioisotope is also eliminated in urine, the urinary bladder being imaged 15 minutes postinjection without any observable kidney accumulation.
Figure 3.
Scintigraphic images of 123I-pLH, 123I-eCG and free 123I distribution in male rats after IV injection. Left panel: 123I-pLH. ; Central panel: 123I-eCG; Right panel: 123INa.
Radioiodinated pLH
Immediatly after IV injection, radiolabeled pLH was seen in highly vascularized organs, such as heart, lungs and liver (figure 3; left panel). They exhibited a rapid clearance in these organs and we determined the t1/2 of 123I-pLH by following its emission in blood at heart level making the assumption that there is no binding of LH in heart (figure 4). A t1/2 value of 2.64 min (95%CL 2.26–3.18) for 123I-pLH was calculated. Accumulation of radioiodinated pLH occurred in the kidneys: 14% of the total injected dose was recovered in the left kidney only ten minutes after injection. When whole body scintigraphic images were examined (figure 3), it appeared that all radioactivity was localized in the renal tract in less than 10 minutes p.i., with very low activity observed in urinary bladder. In the liver, radioactivity appeared fugaciously, and disappeared very rapidly. No radioactivity was present in stomach and intestine until 30 minutes p.i., and thyroid became visible only after 60 minutes p.i. At this time, the different activities observed for the left and the right kidneys could be ascribed to stomach superimposition over kidney. No significant accumulation of radioactivity in genitals nor in any other extravascular compartment was observed.
Figure 4.
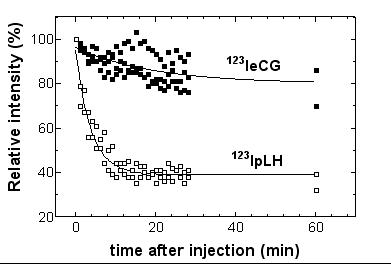
Half-life of 123I-pLH and 123I-eCG in rats by scintigraphy. Quantitation of emission at the level of heart was used to follow residual concentrations of radioiodinated hormones in blood. Half-life of 123I-pLH was determined by non-linear regression of relative intensities as a function of time.
Radioiodinated eCG
Immediately after injection, radiolabeled eCG was seen in the heart and lungs, with a level of activity remaining unchanged over 1 hour (figure 3; central panel) before slowly decreasing until 10 hours (not shown). At this time, these organs still exhibited about 10% of the injected radioactivity dose. Intensity of 123I emission was not superior at the level of liver compared to the lungs, indicating that there was no significant fixation in this organ. No radioactivity was observed in the kidneys, nor in the urinary bladder. No other extravascular compartment accumulated the radiolabeled compound, neither thyroid gland nor genitals.
Discussion
The liver has been shown to bind glycoproteins through its hepatocyte asialoglycoprotein receptor by recognizing terminal Gal residues after removal of sialic acid from their SiaAc-Gal-GlcNAc-Man complex carbohydrate chains. In contrast to FSH and CG, LH from different species bear very low amounts of sialic acid and bear sulfated-GalNAc-GlcNac-Man instead. Specific receptors for this type of chain has been described in liver [4] and they have been proposed to be responsible for the very fast elimination of pituitary native LH compared to the sialylated recombinant LH produced in CHO cells.
Native pituitary LH from various species disappear rapidly from the circulation and their t1/2 during this first phase of elimination have been found to be approximately 15 min for hLH in the human [17,18] and as low as low as 1.2 min for bovine LH in the rat [19]. In agreement with these previous data, we found that the initial phase of elimination of porcine LH from the circulation in the pig and rat is extremely fast. In pig, a half-life (t1/2) of less than 5 minutes was observed for pLH despite we injected the hormone in arteria to avoid hepatic first-pass effect. Since pLH is the homologous hormone, it is likely that this low t1/2 value is meaningful. We expected that the elimination of injected pLH would be slower when hepatic portal circulation was totally derived to vena cava and jugular vein since receptors for sulfated-GalNAc-GlcNac-Man are present in the liver [4]. To our surprise, we found that the initial phase of pLH elimination was not significantly changed by liver shunt and its t1/2 value remained at 5 minutes.
In rat, the t1/2 of pituitary pLH determined by ELISA after bolus injection (6 min) is quite similar to that found in pig (5 min) and in rat as in pig, eCG did not exhibit any fast elimination phase. The similarity of t1/2 of pLH in pig and rat prompted us to determine more precisely the route of fast elimination of pLH in the rat which is more amenable to scintigraphic imaging than the pig.
Only about one molecule out of 1000 is radioiodinated in 123I-pLH solution and thus radioactive molecules are most likely mono-iodinated on their tyrosine residue in position 21 of their α-subunit [20]. The substitution of the Tyr α-21 residue of pLH by two cold iodine atoms leads to a moderate decrease in the in-vivo bioactivity of pLH in rats [20]. The substitution by only one iodine atom is expected to have an even lower effect on pLH bioactivity indicating that its mono-iodination does not modify its rate of elimination and consequently does not change its route of elimination.
We determined the t1/2 of 123I-pLH by following its emission in blood at heart level making the assumption that there is no binding of LH in heart. The found t1/2 of 2.6 min is consistent with the values of 1.2 min for 125I-bLH [19] and 6.2 min for unlabeled pLH (this work) in the rat and confirms the very fast elimination of LH molecules from the circulation. The slightly lower t1/2 values obtained with radioiodinated LHs could arise from preferential iodination of basic isoforms which are known to exhibit shorter half-life than acidic isoforms. Alternatively, it could be that acidic isoforms are detected slightly more efficiently than basic ones in ELISA. In any case, 123I-pLH like native LH is rapidly eliminated from blood and scintigraphic imaging of its early distribution has permitted to localize its initial route of elimination.
After injection of 123I-pLH, emission was located in the stomach, intestine and thyroid only after 30 minutes p.i. This indicates that free iodine due to deiodination of the hormone, had no contribution during the initial 30-min period.
The most prominent observation in this study was that 123I-pLH was trapped very rapidly and very efficiently at the level of kidneys not the liver. Free 123I binding to stomach, gut and thyroid after radiolabeled hormone injections arose only after 30 min, much later than after Na123I injection. Since these sites are known sites for iodide binding [21], it is clear that the initial very fast binding at the kidneys is not due to 123I from radiolabeled pLH, but to the capture of 123I-pLH itself.
The rapid renal trapping of 123I-pLH observed in the present study is at variance with the hypothesis that LH is rapidly cleared from circulation by the sulfated-GalNAc-GlcNac-Man receptors of hepatic reticuloendothelial cells [4]. By contrast, our data are in agreement with previous results [22] that showed an increase of the half-life of [3H-methyl]ovine LH in nephrectomized rats relative to intact rats. These authors also showed that LH accumulates in the renal cortex after glomerular filtration and is reabsorbed by tubular epithelia, with subsequent lysosomal catabolism. Half-life of LH had also been shown to increase in castrated nephrectomized rats and sheep [5,6]. Altogether, our results and these data strongly support the view that kidneys play a major role in the fast clearance of LH. Interestingly, there was no significant transfer to urine in the bladder, at least over the first hour after injection. This puzzling observation suggests that LH is not simply filtrated by the glomerulus but must be linked to some binding sites. Bioactive forms of human LH and FSH are found in large quantities in the urine of post-menopausal women indicating that these gonadotropins are filtrated in the kidneys and pass to urine without extensive degradation. In this physiological situation, kidneys are faced to long-lasting presence of a large concentration of hormone and not to a single bolus of it. We thus suggest that the binding sites for LH in the kidney become saturated in post-menopausal women in the permanent presence of large concentrations of LH. In the near future, we will test this hypothesis in the rat model using the scintigraphic approach described here.
No LH/CG receptors have been reported in the kidney, at least in the human species [23]. Binding of 125I-oLH to ovine kidney membrane fractions has been evidenced in vitro but, interestingly, it was not diminished in the presence of a large excess of cold LH (Combarnous Y, unpublished). Thus, this binding cannot be attributed to saturable high-affinity receptors, but rather to low-affinity high-capacity binding sites. Renotropic activity of oLH [24,25] and pLH [26] has been reported and it has been proposed that a carbohydrate moiety of these gonadotropins containing a sulfate group is implicated in this activity [27]. For the time being, the presence of binding site for sulfated carbohydrates has not been demonstrated in the kidney.
In the case of 123I-eCG, the activities measured in the liver and in the lungs were very similar. This indicates that the hormone can be detected in blood in highly vascularized organs without accumulation in a specific organ, explaining its well-known long half-life. We didn't find noticeable radioactivity in the genital tract for both hormones. The number of LH/CG receptors is known to be only 20 000 per Leydig cell [28] which might be unsufficient for efficient scintigraphic detection.
At the molecular level, it is now of interest to find out what are the structural features of the LH molecule, not present in eCG, that are responsible for its very fast trapping by kidneys. Since the carbohydrate moieties of glycoprotein hormones are known to play a prominent role in their pharmacokinetic properties, it will be of particular interest to perform a comparative scintigraphic study of eCG and eLH elimination. Indeed these two hormones are encoded by the same α and β genes and consequently their α and β polypeptide chains are identical. However, they are expressed in the pituitary or placenta respectively and bear very different carbohydrate chains. It has already been shown by Smith et al [29] that eLH has a much lower half-life than eCG and possess sulphated carbohydrate chains in contrast to eCG. These authors also found radioactive eLH mainly in the liver at the end of the 30 min-experiment but they did not study the kinetics of binding to the different organs at shorter times. It would be very interesting to see by scintigraphic imaging whether 123I-eLH like 123I-pLH is rapidly trapped by kidneys before it is by liver. Sulfated carbohydrate chains of pLH also bind to the Macrophage Mannose Receptor (MMR) [30] found not only in macrophages and dendritic cells but also in hepatic endothelial cells and kidney mesangial cells. It is thus of interest not only to determine the balance between the hepatic and renal routes for the elimination of the different gonadotropins from plasma at different times after injection but also to determine the respective roles of the membrane lectins (Gal asialoglycoprotein receptor of Ashwell, sulfated-GalNAc of Baenziger or MMR) involved in this process.
Conclusions
In both the porcine and rat species, the liver is not responsible for the rapid elimination of pLH from the circulation (t1/2 ~3–6 min) compared to eCG (t1/2>5 h) In the rat, 123I-pLH is found to be rapidly trapped at the level of kidneys whereas 123I-eCG is not. Experiments in the two species indicate that the very short circulatory half-life of LH is due to rapid renal trapping and not to elimination by the liver.
List of abbreviations
eCG, equine chorionic gonadotropin; IA, intra-arterial injection; IV, intraveinous injection; p.i., post injection; pLH, porcine luteinizing hormone; oLH, ovine luteinizing hormone; eLH, equine luteinizing hormone.
Authors' contributions
DK and YC performed study design and result analyses and wrote the manuscript. SB elaborated the scintigraphic studies protocols. SB and ALe participated with DL, FL and YC in the scintigraphic result analyses. SB, DK and FL participated in the radiolabeling experiments. SB, HL and FL performed experiments with rats. ALo was in charge of experimental surgery on piglets, with the help of TM and FL who drew piglet biological samples and analysed them. DK and FL analysed rat plasma and urine samples.
Acknowledgments
Acknowledgements
We wish to thank Florence Foulon-Gauze for her appreciated technical assistance and Dr Jean Pelletier for providing us with anti-pLH serum. The authors are grateful to the Région Centre Council for financial support.
Contributor Information
Danièle Klett, Email: Daniele.Klett@tours.inra.fr.
Serge Bernard, Email: Serge.Bernard@tours.inra.fr.
François Lecompte, Email: Francois.Lecompte@tours.inra.fr.
Hervé Leroux, Email: Herve.Leroux@tours.inra.fr.
Thierry Magallon, Email: Thierry.Magallon@tours.inra.fr.
Alain Locatelli, Email: Alain.Locatelli@tours.inra.fr.
Alain Lepape, Email: lepape@med.univ-tours.fr.
Yves Combarnous, Email: combarno@tours.inra.fr.
References
- Bahl OP, Moyle WR. In: Role of carbohydrate in the action of gonadotropins, in Receptors and hormone action. Birnbaumer L, editor. Academic Press: New York, London; 1978. pp. 261–289. [Google Scholar]
- Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246:1461–1467. [PubMed] [Google Scholar]
- Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Fiete D, Srivastava V, Hindsgaul O, Baenziger JU. A hepatic reticuloendothelial cell receptor specific for SO4-4 GalNAc beta 1,4 GlcNAc beta 1,2 Man alpha that mediates rapid clearance of lutropin. Cell. 1991;67:1103–1110. doi: 10.1016/0092-8674(91)90287-9. [DOI] [PubMed] [Google Scholar]
- Gay VL. Decreased metabolism and increased serum concentrations of LH and FSH following nephrectomy of the rat: absence of short-loop regulatory mechanisms. Endocrinology. 1974;95:1582–1588. doi: 10.1210/endo-95-6-1582. [DOI] [PubMed] [Google Scholar]
- De Kretser DM, Atkins RC, Paulsen CA. Role of the kidney in the metabolism of luteinizing hormone. J Endocrinol. 1973;58:425–434. doi: 10.1677/joe.0.0580425. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Liddle RA, Puett D. The metabolism of luteinizing hormone. Plasma clearance, urinary excretion, and tissue uptake. Mol Cell Endocrinol. 1975;3:21–36. doi: 10.1016/0303-7207(75)90029-5. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Papkoff H. Studies on the disappearance of equine chorionic gonadotropin from the circulation in the rat: tissue uptake and degradation. Endocrinology. 1981;109:1242–1247. doi: 10.1210/endo-109-4-1242. [DOI] [PubMed] [Google Scholar]
- Martinuk SD, Manning AW, Black WD, Murphy BD. Effects of carbohydrates on the pharmacokinetics and biological activity of equine chorionic gonadotropin in vivo. Biol Reprod. 1991;45:598–604. doi: 10.1095/biolreprod45.4.598. [DOI] [PubMed] [Google Scholar]
- Lecompte F, Combarnous Y. Enzyme immunoassay (EIA) for equine chorionic gonadotropin/pregnant mare serum gonadotropin (eCG/PMSG) J Immunoassay. 1992;13:483–493. doi: 10.1080/15321819208019830. [DOI] [PubMed] [Google Scholar]
- Maghuin-Rogister G, Closset J, Hennen G. The carboxy-terminal primary structure of the alpha subunit from bovine and porcine luteinizing hormone. FEBS Lett. 1971;13:301–305. doi: 10.1016/0014-5793(71)80246-6. [DOI] [PubMed] [Google Scholar]
- Camous S, Prunier A, Pelletier J. Plasma prolactin, LH: FSH and estrogen excretion patterns in gilts during sexual development. J Anim Sci. 1985;60:1308–1317. doi: 10.2527/jas1985.6051308x. [DOI] [PubMed] [Google Scholar]
- Anouassi A, Combarnous Y, Lecompte F, Cahoreau C, Guillou F. Purification and characterization of luteinizing hormone from the dromedary (Camelus dromedarius) Biochimie. 1987;69:647–654. doi: 10.1016/0300-9084(87)90184-2. [DOI] [PubMed] [Google Scholar]
- Combarnous Y, Henge MH. Equine follicle-stimulating hormone. Purification, acid dissociation, and binding to equine testicular tissue. J Biol Chem. 1981;256:9567–9572. [PubMed] [Google Scholar]
- Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van der Meer JW, Corstens FH. Radiolabeled interleukin-8: specific scintigraphic detection of infection within a few hours. J Nucl Med. 2000;41:463–469. [PubMed] [Google Scholar]
- Veldhuis JD, Guardabasso V, Rogol AD, Evans WS, Oerter KE, Johnson ML, Rodbard D. Appraising the nature of luteinizing hormone secretory events in men. Am J Physiol. 1987;252:E599–E605. doi: 10.1152/ajpendo.1987.252.5.E599. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci U S A. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger JU, Kumar S, Brodbeck RM, Smith PL, Beranek MC. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc Natl Acad Sci U S A. 1992;89:334–338. doi: 10.1073/pnas.89.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarnous Y, Maghuin-Rogister G. Luteinizing hormone. 2. Relative reactivities of tyrosyl residues of the porcine hormone towards iodination. Eur J Biochem. 1974;42:13–19. doi: 10.1111/j.1432-1033.1974.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Blower PJ, Puncher MR, Kettle AG, George S, Dorsch S, Leak A, Naylor LH, O'Doherty MJ. Iodine-123 salmon calcitonin, an imaging agent for calcitonin receptors: synthesis, biodistribution, metabolism and dosimetry in humans. Eur J Nucl Med. 1998;25:101–108. doi: 10.1007/s002590050200. [DOI] [PubMed] [Google Scholar]
- Robinson JP, Derreberry S, Liddle RA, Ascoli M, Puett D. Renal uptake of lutropin. Studies based on electron microscopic autoradiography and nephrectomy. Mol Cell Biochem. 1977;15:63–66. doi: 10.1007/BF01731289. [DOI] [PubMed] [Google Scholar]
- Rao CV. An overview of the past, present, and future of nongonadal LH/hCG actions in reproductive biology and medicine. Semin Reprod Med. 2001;19:7–17. doi: 10.1055/s-2001-13906. [DOI] [PubMed] [Google Scholar]
- Nomura K, Horiba N, Sato Y, Ujihara M, Demura H, Shizume K. Renotropic activity of lutropin: direct stimulation of DNA synthesis of cultured rat renal cortical cells. Biochem Biophys Res Commun. 1988;150:506–510. doi: 10.1016/0006-291x(88)90549-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Higashihara E, Nomura K, Moriyama N, Aso Y, Demura H. The renotropic effect of ovine luteinizing hormone on subtotally nephrectomized rats. Endocrinol Jpn. 1992;39:109–114. doi: 10.1507/endocrj1954.39.109. [DOI] [PubMed] [Google Scholar]
- Nomura K, Ohmura K, Nakamura Y, Horiba N, Shirakura Y, Sato Y, Ujihara M, Ohki K, Shizume K. Porcine luteinizing hormone isoform(s): relationship between their molecular structures, and renotropic versus gonadotropic activities. Endocrinology. 1989;124:712–719. doi: 10.1210/endo-124-2-712. [DOI] [PubMed] [Google Scholar]
- Nomura K, Nakamura Y, Ujihara M, Ohmura K, Toraya S, Horiba N, Demura H. Renotropic and gonadotropic activity in homologous and heterologous hybrids of ovine luteinizing hormone and human chorionic gonadotropin subunits. Acta Endocrinol (Copenh) 1991;125:590–594. doi: 10.1530/acta.0.1250590. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Pakarinen P, Huhtaniemi I. How much LH do the Leydig cells see? J Endocrinol. 2002;175:375–382. doi: 10.1677/joe.0.1750375. [DOI] [PubMed] [Google Scholar]
- Smith P, Bousfield G, Kumar S, Fiete D, Baenziger J. Equine lutropin and chorionic gonadotropin bear oligosaccharides terminating with SO4-4-GalNAc and Sia alpha 2,3 Gal, respectively. J Biol Chem. 1993;268:795–802. [PubMed] [Google Scholar]
- Leteux C, Chai W, Loveless RW, Yuen CT, Uhlin-Hansen L, Combarnous Y, Jankovic M, Maric SC, Misulovin Z, Nussenzweig MC, Feizi T. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewis(a) and Lewis(x) types in addition to the sulfated N-glycans of lutropin. J Exp Med. 2000;191:1117–1126. doi: 10.1084/jem.191.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]