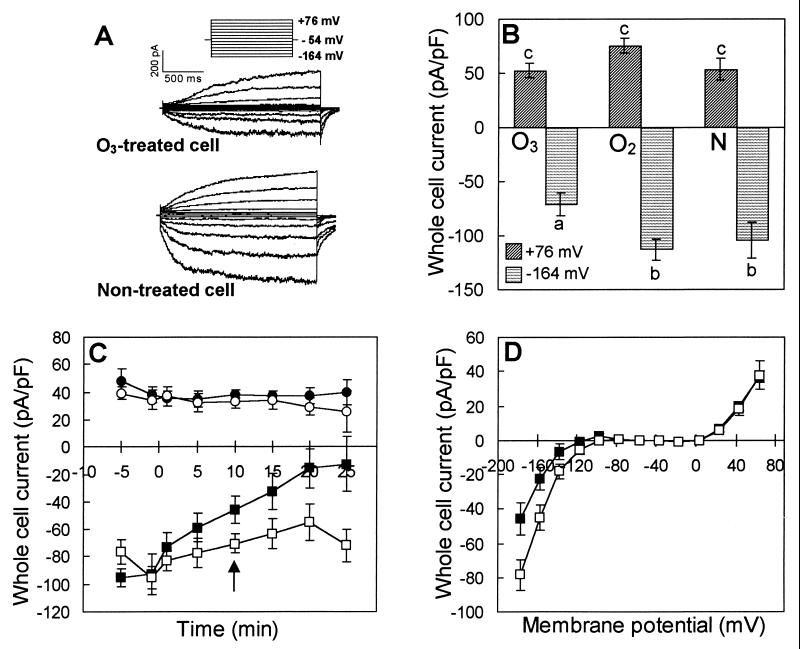
Figure 1.
Ozone exposure of V. faba guard cell protoplasts reduces inward K+ currents. (A) Representative whole cell currents recorded from one O3-treated and one nontreated cell using 100 mM K+ bath and pipette solutions (I). Currents were identified as K+ currents based on their reversal potentials (data not shown) and their characteristic time-activation and voltage-dependence (24). Seal resistance for the cells in A was 2.0 GΩ for the O3-treated cell and 1.2 GΩ for the nontreated cell. (B) Average voltage-activated whole cell K+ currents, normalized for cell size, measured at membrane potentials of +76 mV and −164 mV. For both A and B, protoplasts were exposed to 33–354 μl⋅liter−1 O3 (n = 21) for <5 min in a 300 mM K+-phosphate buffer (see Material and Methods). Controls were protoplasts exposed to O2-treated phosphate buffer (O2, n = 14) or to nontreated phosphate buffer (N, n = 11). Protoplasts were transferred to bath solution I for immediate patch clamping after exposure, and whole cell recordings were performed between 8 and 50 min after exposure, depending on the time required to obtain a whole cell seal. Seal resistance ranged from 0.9 to 4 GΩ for the 46 cells of this experiment and did not differ significantly between treatments (data not shown). Ozone induced a significant (P < 0.01) reduction in inward currents compared with O2- and nontreated protoplasts; there was no significant treatment effect on the outward currents. (C) Voltage-activated whole cell currents measured before and after treatment (treatment at time 0) at membrane potentials of +63 mV for O3-treated (●) or nontreated (○) protoplasts and at −177 mV for O3-treated (■) or nontreated (□) protoplasts. The effects on inward K+ current of O3, time, and O3 treatment by time are significant at the P < 0.05 level. (D) Current-voltage relationships from whole cell measurements recorded 10 min (indicated by arrow in C) after adding O3-treated (■, n = 6) or nontreated buffer (□, n = 8) directly to the bath solution (phosphate buffer described above). Ozone significantly inhibited inward K+ current at voltages ≤−117 mV. For both C and D, the whole cell configuration was obtained with a 10 mM K+ bath solution (II) and a 100 mM K+ pipette solution (II). The bath was thereafter perfused with the phosphate buffer described in Materials and Methods for 3 min, and 200 μl of O3-treated or nontreated phosphate buffer was added to the 1-ml bath dish ≈5 min after the end of perfusion. The initial O3 concentration in the dish ranged from 10 to 30 μl⋅liter−1 and in the presence of the protoplasts the concentration dropped by 75% within 10 s.