Figure 2.
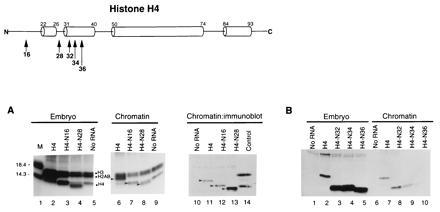
The amino terminal tail of histone H4 is dispensable for assembly into replicating chromatin, but the N-terminal α-helix is important. (A) mRNA encoding epitope-tagged, wild-type (H4; lanes 2, 6, and 11) or N-terminal deletion mutants 1–16 (N16; lanes 3, 7, and 12) and 1–28 (N28; lanes 4, 8, and 13) or no RNA (lanes 5, 9, and 10) were injected into embryos, along with tritiated arginine and lysine to radiolabel proteins and [α-32P]dCTP to label DNA. Embryos were assayed for levels of total newly synthesized radiolabeled histone (lanes 2–5) and for levels of newly synthesized radiolabeled histone incorporated into chromatin (lanes 6–9) by gel electrophoresis followed by fluorography. Filled squares indicate positions of labeled endogenous core histones; arrows indicate positions of labeled epitope-tagged, wild type and mutant histones. The level of epitope-tagged, wild-type and mutant histones were determined by immunoblotting (lanes 11–14). Lane 1 is a molecular weight marker from BRL (Mr 14,300; 18.4 kDa); lane 14 includes radiolabeled wild-type histones H3 and H4. (B) mRNA encoding epitope-tagged, wild-type (H4; lanes 2 and 7) or N-terminal deletion mutants NΔ1-32 (N32; lanes 3 and 8), NΔ1-34 (N36; lanes 4 and 9), NΔ1-36 (N36; lanes 5 and 10), or no RNA (lanes 1 and 6) were injected as above. Levels of epitope-tagged, wild-type and mutant histones in embryos (lanes 1–5) and chromatin (lanes 6–10) were determined by immunoblotting. The immunoreactive bands above those due to the epitope-tagged H4 correspond to non-histone proteins that crossreact with the FLAG epitope that copurify with nucleosomes.
