Abstract
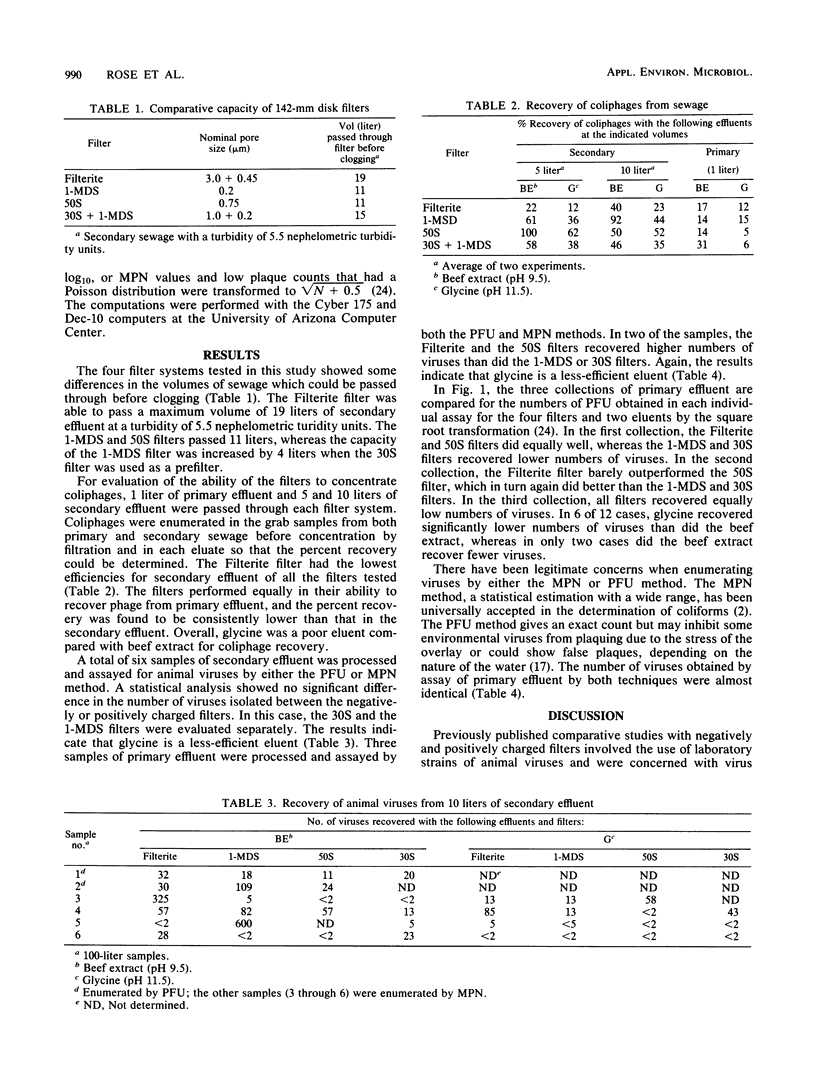
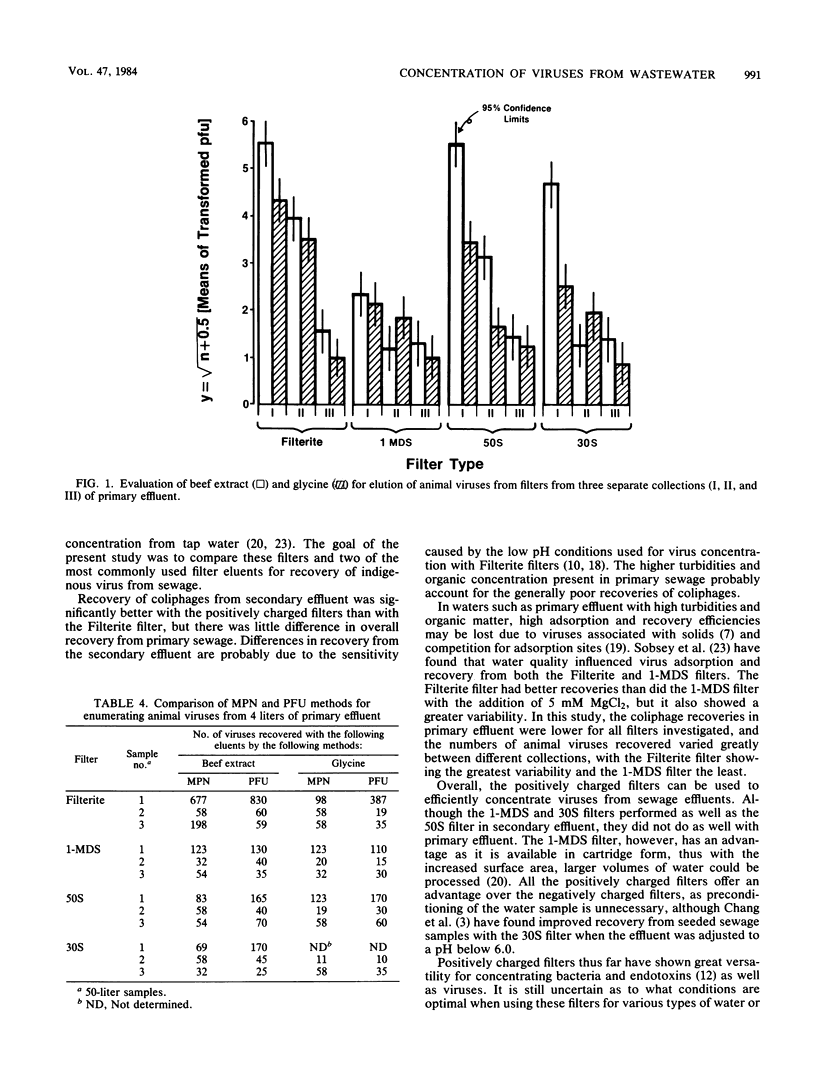
The 1-MDS Virosorb filter and the 50S and 30S Zeta-plus filters, all with a net positive charge, were compared with the negatively charged Filterite filter for concentration of naturally occurring coliphages and animal viruses from sewage effluent. When Filterite filters were used, the effluent was adjusted to pH 3.5 and AlCl3 was added before filtration to facilitate virus adsorption. No adjustment was required with the positively charged filters. Sets of each filter type were eluted with 3% beef extract (pH 9.5) or eluted with 0.05 M glycine (pH 11.5). A maximum volume of 19 liters could be passed through 142-mm diameter Filterite filters before clogging, whereas only 11, 11, and 15 liters could be passed through the 1-MDS, 50S, and 30S filters, respectively. For equal volumes passed through the filters, coliphage recoveries were 14, 15, 18, and 37% in primary effluent and 40, 97, 50, and 46% in secondary effluent for the Filterite , 1-MDS, 50S, and 30S filters, respectively. No statistically significant difference was observed in the recovery of animal viruses among the filters from secondary effluent, whereas in the Filterite and 50S filters, higher numbers of viruses from primary effluent were recovered than in the 1-MDS and 30S filters in two of three collections. Glycine was found to be a less-efficient eluent than beef extract in the recovery of naturally occurring viruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. T., Farrah S. R., Bitton G. Positively charged filters for virus recovery from wastewater treatment plant effluents. Appl Environ Microbiol. 1981 Nov;42(5):921–924. doi: 10.1128/aem.42.5.921-924.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Bitton G. Elution of poliovirus adsorbed to membrane filters. Appl Environ Microbiol. 1978 Dec;36(6):982–984. doi: 10.1128/aem.36.6.982-984.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Farrah S. R., Goyal S. M., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl Environ Microbiol. 1978 Mar;35(3):540–548. doi: 10.1128/aem.35.3.540-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Simple method for concentration of bacteria from large volumes of tap water. Appl Environ Microbiol. 1980 Nov;40(5):912–916. doi: 10.1128/aem.40.5.912-916.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Hanssen H., Gerba C. P. Simple method for the concentration of influenza virus from allantoic fluid on microporous filters. Appl Environ Microbiol. 1980 Mar;39(3):500–504. doi: 10.1128/aem.39.3.500-504.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Zerda K. S., Gerba C. P. Concentration of coliphages from large volumes of water and wastewater. Appl Environ Microbiol. 1980 Jan;39(1):85–91. doi: 10.1128/aem.39.1.85-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou K., Gerba C. P., Goyal S. M., Zerda K. S. Capture of latex beads, bacteria, endotoxin, and viruses by charge-modified filters. Appl Environ Microbiol. 1980 Nov;40(5):892–896. doi: 10.1128/aem.40.5.892-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswick B. H., Gerba C. P., Goyal S. M. Occurrence of enteroviruses in community swimming pools. Am J Public Health. 1981 Sep;71(9):1026–1030. doi: 10.2105/ajph.71.9.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan K. B., Rees G. E., Seeley N. D., Primrose S. B. Rapid concentration of bacteriophages from large volumes of freshwater: evaluation of positively charged, microporous filters. J Virol Methods. 1980;1(2):87–97. doi: 10.1016/0166-0934(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Ho H. H., Riggs J. L., Lennette E. H. Comparative sensitivity of various cell culture systems for isolation of viruses from wastewater and fecal samples. Appl Environ Microbiol. 1978 Sep;36(3):480–486. doi: 10.1128/aem.36.3.480-486.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley N. D., Primrose S. B. Concentration of bacteriophages from natural waters. J Appl Bacteriol. 1979 Feb;46(1):103–116. doi: 10.1111/j.1365-2672.1979.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Glass J. S. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980 Aug;40(2):201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


