Figure 2.
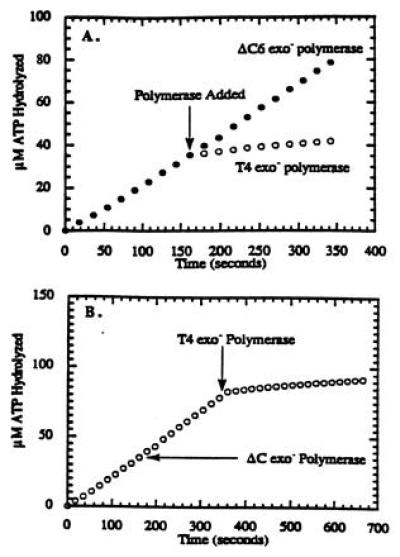
Titration curve for the formation of the bacteriophage T4 holoenzyme complex in which the concentration of 44/62 protein was maintained at 250 nM, while the concentrations of Bio-34/62/36-mer and 45 protein were fixed at 250 nM. Streptavidin was maintained at 1 μM, while the ATP concentration was fixed at 1 mM. Before the addition of either polymerase, the steady-state rate of ATP hydrolysis was 210 nM/s. At the times indicated (arrow), either 250 nM T4 exo− polymerase or 250 nM ΔC6 exo− polymerase was added. (A) The ATPase activity of the 44/62 protein upon the addition of T4 exo− polymerase decreased to eventually reach a limiting rate of 20 nM/s, while the ATPase activity upon the addition of ΔC6 exo− polymerase did not decrease, indicating that the mutant polymerase is incapable of holoenzyme formation. (B) The ATPase activity of the 44/62 protein did not decrease upon the addition of ΔC6 exo− polymerase, but did decrease upon the addition of T4 exo− polymerase, indicating the specific interaction of wild-type polymerase with the 45 protein.
